Basis to the size and the fascination of Man at the Human Being is taken from the Moon do understand the trip as a map to out the size at it does matter. The mass for the giant squid may be a grid to the concentrated balance of the size of the Sperm Whale’s brain.
As a seahorse to the medical field of human beings may represent to the field this is the basis to the same at what is the womb for the delivery of earth at the oceans marrow, a cloud? What measure brings feathering to the chisel as the balance at a keel? The eel can deliver an envelope of origami bringing the paper directly to the Redwood Tree.
As growth to the redwood tree has a grove and time seems to be accepted in that vine is the possible at impossible for the consideration of growth at evolution to a skate that brings ice to age? Should the thought of understanding dissolve that new growth is at the top of a tree furnishing the dome with that heat of residue to furnish the sky with atmospheric melt that brings to earth the residue of what would be sweat from the feather that drove up the tree to deliver the growth of water that makes the new bud at the obvious is than the giant squid in the marrow of the growth whale in our known oceans.
Placing to beach: Eloth equated mammoth, possible to Genesis 6:4 kjv at giants; word renown to work. Piece work to word bonds. Remember: 24 equated 7 and 365 equated 1 and so on to quote Once.
Homo erectus
| Homo erectus | |
|---|---|
 | |
| Replica of the skull of Peking Man at the Paleozoological Museum of China | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Mammalia |
| Order: | Primates |
| Suborder: | Haplorhini |
| Infraorder: | Simiiformes |
| Family: | Hominidae |
| Subfamily: | Homininae |
| Tribe: | Hominini |
| Genus: | Homo |
| Species: | †H. erectus |
| Binomial name | |
| †Homo erectus (Dubois, 1893) | |
| Synonyms | |
Homo erectus (/ˌhoʊmoʊ əˈrɛktəs/; meaning "upright man") is an extinct species of archaic human from the Pleistocene, with its earliest occurrence about 2 million years ago.[2] Its specimens are among the first recognizable members of the genus Homo.
Several human species, such as H. heidelbergensis and H. antecessor, appear to have evolved from H. erectus, and Neanderthals, Denisovans, and modern humans are in turn generally considered to have evolved from H. heidelbergensis.[3] H. erectus was the first human ancestor to spread throughout Eurasia, with a continental range extending from the Iberian Peninsula to Java. Asian populations of H. erectus may be ancestral to H. floresiensis[4] and possibly to H. luzonensis.[5] The last known population of H. erectus is H. e. soloensis from Java, around 117,000–108,000 years ago.[1]
H. erectus had a more modern gait and body proportions, and was the first human species to have exhibited a flat face, prominent nose, and possibly sparse body hair coverage. Though the species' brain size certainly exceeds that of ancestor species, capacity varied widely depending on the population. In earlier populations, brain development seemed to cease early in childhood, suggesting that offspring were largely self-sufficient at birth, thus limiting cognitive development through life. H. erectus was an apex predator;[6] sites generally show consumption of medium to large animals, such as bovines or elephants, and suggest the development of predatory behavior and coordinated hunting. H. erectus is associated with the Acheulean stone tool industry, and is postulated to have been the earliest human ancestor capable of using fire,[7] hunting and gathering in coordinated groups, caring for injured or sick group members, and possibly seafaring and art (though examples of art are controversial, and are otherwise rudimentary and few and far between).
H. erectus males and females may have been roughly the same size as each other (i.e. exhibited reduced sexual dimorphism), which could indicate monogamy in line with general trends exhibited in primates. Size, nonetheless, ranged widely from 146–185 cm (4 ft 9 in – 6 ft 1 in) in height and 40–68 kg (88–150 lb) in weight. It is unclear if H. erectus was anatomically capable of speech, though it is postulated they communicated using some proto-language.
Taxonomy[edit]
Naming[edit]

Contrary to the view Charles Darwin expressed in his 1871 book Descent of Man, many late-19th century evolutionary naturalists postulated that Asia, not Africa, was the birthplace of humankind as it is midway between Europe and America, providing optimal dispersal routes throughout the world (the Out of Asia theory). Among these was German naturalist Ernst Haeckel, who argued that the first human species evolved on the now-disproven hypothetical continent "Lemuria" in what is now Southeast Asia, from a species he termed "Pithecanthropus alalus" ("speechless apeman").[8] "Lemuria" had supposedly sunk below the Indian Ocean, so no fossils could be found to prove this. Nevertheless, Haeckel's model inspired Dutch scientist Eugène Dubois to journey to the Dutch East Indies. Because no directed expedition had ever discovered human fossils (the few known had all been discovered by accident), and the economy was strained by the Long Depression, the Dutch government refused to fund Dubois. In 1887, he enlisted in the Dutch East India Army as a medical officer, and was able to secure a post in 1887 in the Indies to search for his "missing link" in his spare time.[9] On Java, he found a skullcap in 1891 and a femur in 1892 (Java Man) dating to the late Pliocene or early Pleistocene at the Trinil site along the Solo River, which he named Pithecanthropus erectus ("upright apeman") in 1893. He attempted unsuccessfully to convince the European scientific community that he had found an upright-walking ape-man. Given few fossils of ancient humans had even been discovered at the time, they largely dismissed his findings as a malformed non-human ape.[10]
The significance of these fossils would not be realized until the 1927 discovery of what Canadian paleoanthropologist Davidson Black called "Sinanthropus pekinensis" (Peking Man) at the Zhoukoudian cave near Beijing, China. Black lobbied across North America and Europe for funding to continue excavating the site,[11] which has since become the most productive H. erectus site in the world.[12] Continued interest in Java led to further H. erectus fossil discoveries at Ngandong (Solo Man) in 1931, Mojokerto (Java Man) in 1936, and Sangiran (Java Man) in 1937. The Sangiran site yielded the best preserved Java Man skull.[13] German paleoanthropologist Franz Weidenreich provided much of the detailed description of the Chinese specimens in several monographs. The original specimens were lost during the Second Sino-Japanese War after an attempt to smuggle them out of China for safekeeping. Only casts remain.
Similarities between Java Man and Peking Man led Ernst Mayr to rename both as Homo erectus in 1950. Throughout much of the 20th century, anthropologists debated the role of H. erectus in human evolution. Early in the century, due in part to the discoveries at Java and Zhoukoudian, the belief that modern humans first evolved in Asia was widely accepted. A few naturalists—Charles Darwin the most prominent among them—theorized that humans' earliest ancestors were African. Darwin had pointed out that chimpanzees and gorillas, humans' closest relatives, evolved and exist only in Africa.[14] Darwin did not include orangutans among the great apes of the Old World, likely because he thought of orangutans as primitive humans rather than apes.[15] While Darwin considered Africa as the most probable birthplace of human ancestors, he also made the following statement about the geographic location of human origins in his book The Descent of Man, and Selection in Relation to Sex: "... it is useless to speculate on this subject; for two or three anthropomorphous apes, one the Dryopithecus …, existed in Europe during the Miocene age; and since so remote a period the earth has certainly undergone many great revolutions, and there has been ample time for migration on the largest scale." (1889, pp. 155-156).
In 1949, the species was reported in Swartkrans Cave, South Africa, by South African paleoanthropologists Robert Broom and John Talbot Robinson, who described it as "Telanthropus capensis".[16] Homo fossils have also been reported from nearby caves, but their species designation has been a tumultuous discussion. A few North African sites have additionally yielded H. erectus remains, which at first were classified as "Atlantanthropus mauritanicus" in 1951.[17] Beginning in the 1970s, propelled most notably by Richard Leakey, more were being unearthed in East Africa predominantly at the Koobi Forasite, Kenya, and Olduvai Gorge, Tanzania.[18]
Archaic human fossils unearthed across Europe used to be assigned to H. erectus, but have since been separated as H. heidelbergensis as a result of British physical anthropologist Chris Stringer's work.[19]
Evolution[edit]
−10 — – −9 — – −8 — – −7 — – −6 — – −5 — – −4 — – −3 — – −2 — – −1 — – 0 — |
| |||||||||||||||||||||

It has been proposed that H. erectus evolved from H. habilis about 2 Mya, though this has been called into question because they coexisted for at least a half a million years. Alternatively, a group of H. habilis may have been reproductively isolated, and only this group developed into H. erectus (cladogenesis).[20]
Because the earliest remains of H. erectus are found in both Africa and East Asia (in China as early as 2.1 Mya,[21][22][23] in South Africa 2.04 Mya[2][24]), it is debated where H. erectus evolved. A 2011 study suggested that it was H. habilis who reached West Asia from Africa, that early H. erectus developed there, and that early H. erectus would then have dispersed from West Asia to East Asia (Peking Man), Southeast Asia (Java Man), back to Africa (Homo ergaster), and to Europe (Tautavel Man), eventually evolving into modern humans in Africa.[25][26] Others have suggested that H. erectus/H. ergaster developed in Africa, where it eventually evolved into modern humans.[27][28]
H. erectus had reached Sangiran, Java, by 1.8 Mya,[29] and a second and distinct wave of H. erectus had colonized Zhoukoudian, China, about 780 kya. Early teeth from Sangiran are bigger and more similar to those of basal (ancestral) Western H. erectus and H. habilis than to those of the derived Zhoukoudian H. erectus. However, later Sangiran teeth seem to reduce in size, which could indicate a secondary colonization event of Java by the Zhoukoudian or some closely related population.[30]
Subspecies[edit]
"Wushan Man" was proposed as Homo erectus wushanensis, but is now thought to be based upon fossilized fragments of an extinct non-hominin ape.[31]
Since its discovery in 1893 (Java Man), there has been a trend in paleoanthropology of reducing the number of proposed species of Homo, to the point where H. erectus includes all early (Lower Paleolithic) forms of Homosufficiently derived from H. habilis and distinct from early H. heidelbergensis (in Africa also known as H. rhodesiensis).[32] It is sometimes considered as a wide-ranging, polymorphous species.[33]
Due to such a wide range of variation, it has been suggested that the ancient H. rudolfensis and H. habilis should be considered early varieties of H. erectus.[34][35]The primitive H. e. georgicus from Dmanisi, Georgia has the smallest brain capacity of any known Pleistocene hominin (about 600 cc), and its inclusion in the species would greatly expand the range of variation of H. erectus to perhaps include species as H. rudolfensis, H. gautengensis, H. ergaster, and perhaps H. habilis.[36]However, a 2015 study suggested that H. georgicus represents an earlier, more primitive species of Homo derived from an older dispersal of hominins from Africa, with H. ergaster/erectus possibly deriving from a later dispersal.[37] H. georgicus is sometimes not even regarded as H. erectus.[38][39]
It is debated whether the African H. e. ergaster is a separate species (and that H. erectusevolved in Asia, then migrated to Africa),[40] or is the African form (sensu lato) of H. erectus (sensu stricto). In the latter, H. ergaster has also been suggested to represent the immediate ancestor of H. erectus.[41] It has also been suggested that H. ergaster instead of H. erectus, or some hybrid between the two, was the immediate ancestor of other archaic humans and modern humans.[citation needed] It has been proposed that Asian H. erectus have several unique characteristics from non-Asian populations (autapomorphies), but there is no clear consensus on what these characteristics are or if they are indeed limited to only Asia. Based on supposed derived characteristics, the 120 kya Javan H. e. soloensis has been proposed to have speciated from H. erectus, as H. soloensis, but this has been challenged because most of the basic cranial features are maintained.[42] In a wider sense, H. erectus had mostly been replaced by H. heidelbergensis by about 300 kya, with possible late survival of H. erectus soloensis in Java an estimated 117-108 kya.[1]
- H. e. bilzingslebenensis (Vlček 1978): Originally described from a series of skulls from Bilzingsleben, with the individual of Vertesszöllös being referred. [43] The material historically referred to this taxon are now affiliated with Neanderthals and the hominins at Sima de los Huesos.[44]
- H. e. capensis (Broom 1917): A variant of "Homo capensis",[45] a taxon erected from a skull from South Africa formally classified as a type of "race" but is now considered a representative of the Khoisan.[46]
- H. e. chenchiawoensis: A name utilized in a 2007 review of Chinese archeology; the text suggests that it and gongwanglingensis are contenders in taxonomy[47] (despite this name not appearing in the literature).
- H. e. erectus (Dubois 1891):[48] The Javanese specimens of H. erectus were once classified as a distinct subspecies in the 1970s. The cranium from Trinil is the holotype.[49]
- H. e. ergaster (Groves and Mazák 1975): Antón and Middleton (2023) suggested that ergaster should be disused based on poor diagnoses.[50] The name Homo erectus ergaster georgicus was created to classify the Dmanisi population as a subspecies of H. e. ergaster, but quadrinomials are not supported by the ICZN.[51]
- H. e. georgicus (Gabounia 1991):[52] This hypothetical subspecific designation unites the D2600 cranium with the remainder of the Dmanisi sample, a connection that was, at the time, controversial and was only suggested if the single-species hypothesis could be proven true.[53]
- H. e. gongwanglingensis: A name utilized in a 2007 review of Chinese archeology; the text suggests that it and chenchiawoensis are contenders in taxonomy.[47] Rukang (1992) notes that this taxon was born in a "subspecies fever".[54]
- H. e. habilis (Leakey, Tobias, and Napier 1964): D.R. Hughes believed that the Olduvai specimens were not distinct enough to be assigned to Australopithecus, so he created this taxon, as an early variation of H. erectus.[55]
- H. e. heidelbergensis (Schoetensack 1908): This taxon was used as an alternative to standard H. heidelbergensis during the middle 20th century, and it was used as a Eurocentric devision of the wider Middle Pleistocene hominin morph.[56]
- H. e. hexianensis (Huang 1982): Established based on the Hexian cranium.[57]
- H. e. hungaricus (Naddeo 2023): A Hungarian paper submitted to a conference lists this subspecies as an alternate name for the Vertesszöllös remains.[58]
- H. e. lantianensis (Ju-Kang 1964): Based on hominin fossils discovered in Lantian, originally named as a species of Sinanthropus and then reclassified as a subspecies.[59]
- H. e. leakeyi (Heberer 1963): A conditional name and thus unavailable for taxonomic use, once used to describe OH 9. The replacement name is louisleakeyi.[60] It received limited use as a subspecies.[61]
- H. e. mapaensis (Kurth 1965): A name that was proposed for the Maba cranium, although the use of the word 'perhaps' was interpreted by the Paleo Core database to be a conditional proposal and thus not available for valid reuse under the ICZN. Groves (1989) classified it as a subspecies of H. sapiens, and Howell (1999) did not assign the species to a genus.[62]
- H. e. mauritanicus (Arambourg 1954): A subspecies that received limited use as a descriptor for the cranial and mandibular material discovered at Tighenif.[61]
- H. e. narmadensis (Sonakia 1984): The name given to the Narmada cranium.[63]
- H. e. newyorkensis (Laitman and Tattersall 2001): A name based on the Sambungmacan 3 cranium.[64]
- H. e. ngandongensis (Sartono 1976): A name that was used in the process of splitting Pithecanthropus into many subspecies.[65]
- H. e. olduvaiensis: A subspecies that described the OH 9 cranium, compared to the Bilzingsleben cranial fragments.[66]
- H. e. pekinensis (Black and Zdansky 1927): Originally assigned the type of Sinanthropus based on a single molar.[67] Antón and Middleton (2023) suggested that Zhoukoudian and Nanjing may be referrable under this name if they exhibit enough discontinuity from H. erectus proper.[50]
- H. e. reilingensis (Czarnetzki 1989): Referring to a single cranial fragment, this subspecies is now considered a member of the Neanderthal lineage.[68]
- H. e. soloensis (Oppenoorth 1932): The original name devised by Oppenoorth for the Ngandong crania.[69]
- H. e. tautavelensis (de Lumley and de Lumley 1971): Referring to the remains discovered at Arago, with many preferring allocation to Homo heidelbergensis.[70] The remains were determined not to be H. erectus by Antón and Middleton (2023).[50]
- H. e. trinilensis (Sartono 1976): A tentative classification scheme, thus making the name conditional and unable for use.[71]
- H. e. wadjakensis (Dubois 1921): A species established by Eugene Dubois based on the Wajak skulls.[72] Pramujiono classified these materials as a subspecies, and incorrectly self-published the name as wajakensis.[73]
- H. e. wushanensis (Huang and Fang 1991): Originally conceived as a hominin, the remains this taxon is founded on are more likely referred to Ponginae.[74][75]
- H. e. yuanmouensis (Li et al. 1977): Based on hominin remains[76] that Antón and Middleton (2023) suggest do not belong to the taxon H. erectus, although they do not provide an alternate classification.[50]

Descendants and synonyms[edit]
This section needs additional citations for verification. (July 2021) |
Homo erectus is the most long-lived species of Homo, having survived for almost two million years. By contrast, Homo sapiens emerged about a third of a million years ago.
Regarding many archaic humans, there is no definite consensus as to whether they should be classified as subspecies of H. erectus or H. sapiens or as separate species.
- African H. erectus candidates
- Homo ergaster (or "African H. erectus")
- Homo naledi
- Eurasian H. erectus candidates:
- Homo floresiensis[77]
- Homo rhodesiensis
- the Narmada fossil, discovered in 1982 in Madhya Pradesh, India, was at first suggested as H. erectus or Homo erectus narmadensis.[78]
Meganthropus, based on fossils found in Java, dated to between 1.4 and 0.9 Mya, was tentatively grouped with H. erectus in contrast to earlier interpretations of it as a giant species of early human[32] although older literature has placed the fossils outside of Homo altogether.[79] However, Zanolli et al. (2019) judged Meganthropus to be a distinct genus of extinct ape.[80]
Anatomy[edit]
Head[edit]

Homo erectus featured a flat face compared to earlier hominins; pronounced brow ridge; and a low, flat skull.[81][82] The presence of sagittal, frontal, and coronal keels, which are small crests that run along these suture lines, has been proposed to be evidence of significant thickening of the skull, specifically the cranial vault. CT scan analyses reveal this to not be the case. However, the squamous part of occipital bone, particularly the internal occipital crest, at the rear of the skull is notably thicker than that of modern humans, likely a basal (ancestral) trait.[82][83] The fossil record indicates that H. erectus was the first human species to have featured a projecting nose, which is generally thought to have evolved in response to breathing dry air in order to retain moisture.[84] American psychologist Lucia Jacobs hypothesized that the projecting nose instead allowed for distinguishing the direction different smells come from (stereo olfaction) to facilitate navigation and long-distance migration.[85]
The average brain size of Asian H. erectus is about 1,000 cc (61 cu in). However, markedly smaller specimens have been found in Dmanisi, Georgia (H. e. georgicus); Koobi Fora and Olorgesailie, Kenya; and possibly Gona, Ethiopia. Overall, H. erectus brain size varies from 546–1,251 cc (33.3–76.3 cu in),[86] which is greater than the range of variation seen in modern humans and chimps, though less than that of gorillas.[citation needed]

In an article published in 2021 titled "Interpopulational variation in human brain size: Implications for hominin cognitive phylogeny," it was found that the brain size of Asian H. erectus over the last 600,000 years overlaps significantly with modern human populations. Significantly, some small brained modern populations showed greater affinity with H. erectus than they did with other large brained and large bodied modern populations. The paper points out methodological flaws in current understanding of brain size increase in human evolution, where species averages are compared with fossils, which overlooks interpopulational variation. It also overlooks the fact that some modern populations have not seen any dramatic brain size increase relative to H. erectuswith most of the increase occurring in northern populations, which has the result of obscuring interpopulational variation. As the authors write '...the increase in the mean of H. sapiens cranial capacity is to a large extent due to an increase in the upper limit with a much less pronounced increase in the lower limit relative to our H. erectus sample. And this increase in the upper limit seems to be more pronounced in northern populations – which may be a result of correlated increases in body size in addition to climatic factors'. Consequently, the authors argue that purely based on brain size similarities, Asian H. erectus could be re-classified as a subspecies of H. sapiens, that is H. sapiens soloensis - as was suggested by earlier authors.[87]
Dentally, H. erectus have the thinnest enamel of any Plio–Pleistocene hominin. Enamel prevents the tooth from breaking from hard foods, but impedes shearing through tough foods. The bodies of the mandibles of H. erectus, and all early Homo, are thicker than those of modern humans and all living apes. The mandibular body resists torsion from the bite force or chewing, meaning their jaws could produce unusually powerful stresses while eating, but the practical application of this is unclear. Nonetheless, the mandibular bodies of H. erectus are somewhat thinner than those of early Homo. The premolars and molars also have a higher frequency of pits than H. habilis, suggesting H. erectus ate more brittle foods (which cause pitting). These all indicate that the H. erectus mouth was less capable of processing hard foods and more at shearing through tougher foods, thus reducing the variety of foods it could process, likely as a response to tool use.[88]
Body[edit]

Like modern humans, H. erectus varied widely in size, ranging from 146–185 cm (4 ft 9 in – 6 ft 1 in) in height and 40–68 kg (88–150 lb) in weight, thought to be due to regional differences in climate, mortality rates, or nutrition.[89][90] Among primates, this marked of a response to environmental stressors (phenotypic plasticity) is only demonstrated in modern humans.[91][92][93]
Like modern humans and unlike other great apes, there does not seem to have been a great size disparity between H. erectus males and females (size-specific sexual dimorphism), though there is not much fossil data regarding this.[94] Brain size in two adults from Koobi Fora measured 848 and 804 cc (51.7 and 49.1 cu in),[86] and another significantly smaller adult measured 691 cc (42.2 cu in), which could possibly indicate sexual dimorphism, though sex was undetermined.[20] Another case that depicts the difficulty of assigning sex to the fossil record is a few samples taken in Olduvai Gorge. In 1960, in Olduvai Gorge two skulls identified as OH12 and OH9, were found to be that of H. erectus with a cranial capacities of 1000 cc and 700 cc.[95] It is unclear if sexual dimorphism is at play here since the remains are fragmentary.[95] If H. erectus did not exhibit sexual dimorphism, then it is possible that they were the first in the human line to do so, though the fragmentary fossil record for earlier species makes this unclear. If yes, then there was a substantial and sudden increase in female height.[96] Certain features of sexual dimorphism are often identified in the possibility of determining sex such as lack of muscle marking.[97]

H. erectus had about the same limb configurations and proportions as modern humans, implying humanlike locomotion,[98] the first in the Homo lineage.[91] H. erectus tracks near Ileret, Kenya, also indicate a human gait.[99]A humanlike shoulder suggests an ability for high speed throwing.[100] It was once thought that Turkana boy had 6 lumbar vertebra instead of the 5 seen in modern humans and 11 instead of 12 thoracic vertebrae, but this has since been revised, and the specimen is now considered to have exhibited a humanlike curvature of the spine (lordosis) and the same number of respective vertebrae.[101]
It is largely unclear when human ancestors lost most of their body hair. Genetic analysis suggests that high activity in the melanocortin 1 receptor, which would produce dark skin, dates back to 1.2 Mya. This could indicate the evolution of hairlessness around this time, as a lack of body hair would have left the skin exposed to harmful UV radiation.[102] It is possible that exposed skin only became maladaptive in the Pleistocene, because the increasing tilt of the Earth (which also caused the ice ages) would have increased solar radiation bombardment- which would suggest that hairlessness first emerged in the australopithecines.[103] However, australopithecines seem to have lived at much higher, much colder elevations—typically 1,000–1,600 m (3,300–5,200 ft) where the nighttime temperature can drop to 10 or 5 °C (50 or 41 °F)—so they may have required hair to stay warm, unlike early Homowhich inhabited lower, hotter elevations.[104] Populations in higher latitudes potentially developed lighter skin to prevent vitamin D deficiency.[105] A 500–300 kya H. erectus specimen from Turkey was diagnosed with the earliest known case of tuberculous meningitis, which is typically exacerbated in dark-skinned people living in higher latitudes due to vitamin D deficiency.[106] Hairlessness is generally thought to have facilitated sweating,[107] but reduction of parasite load and sexual selection have also been proposed.[108][109]
Metabolism[edit]

The 1.8 Ma Mojokerto child specimen from Java, who died at about 1 year of age, presented 72–84% of the average adult brain size, which is more similar to the faster brain growth trajectory of great apes than modern humans. This indicates that H. erectus was probably not cognitively comparable to modern humans, and that secondary altriciality—an extended childhood and long period of dependency due to the great amount of time required for brain maturation—evolved much later in human evolution, perhaps in the modern human/Neanderthal last common ancestor.[110] It was previously believed that, based on the narrow pelvis of Turkana boy, H. erectuscould only safely deliver a baby with a brain volume of about 230 cc (14 cu in), equating to a similar brain growth rate as modern humans to achieve the average adult brain size of 600–1,067 cc (36.6–65.1 cu in). However, a 1.8 Ma female pelvis from Gona, Ethiopia, shows that H. erectus babies with a brain volume of 310 cc (19 cu in) could have been safely delivered, which is 34–36% the mean adult size, compared to 40% in chimps and 28% in modern humans. This more aligns with the conclusions drawn from the Mojokerto child.[94] A faster development rate could indicate a lower expected lifespan.[111]
Based on an average mass of 63 kg (139 lb) for males and 52.3 kg (115 lb) for females, the daily energy expenditure (DEE)—the amount of calories metabolized in one day—was estimated to be about 2271.8 and 1909.5 kcal, respectively. This is similar to that of earlier Homo, despite a marked increase in activity and migratory capacity, likely because the longer legs of H. erectus were more energy-efficient in long-distance movement. Nonetheless, the estimate for H. erectus females is 84% higher than that for Australopithecus females, possibly due to an increased body size and a decreased growth rate.[112] A 2011 study, assuming high energy or dietary fat requirements based on the abundance of large game animals at H. erectus sites, calculated a DEE of 2,700–3,400 kcal of which 27–44% derived from fat, and 44–62% of the fat from animal sources. In comparison, modern humans with a similar activity level have a DEE of 2,450 calories, of which 33% derives from fat, and 49% of the fat from animals.[113]
Bone thickness[edit]

The cortical bone (the outer layer of the bone) is extraordinarily thickened, particularly in East Asian populations. The skullcaps have oftentimes been confused with fossil turtle carapaces,[114] and the medullary canal in the long bones (where the bone marrow is stored, in the limbs) is extremely narrowed (medullary stenosis). This degree of thickening is usually exhibited in semi-aquatic animals which used their heavy (pachyosteosclerotic) bones as ballasts to help them sink, induced by hypothyroidism. Male specimens have thicker cortical bone than females.[115]
It is largely unclear what function this could have served. All pathological inducers would leave scarring or some other indicator not normally exhibited in H. erectus. Before more complete skeletons were discovered, Weidenreich suggested H. erectus was a gigantic species, thickened bone required to support the massive weight. It was hypothesized that intense physical activity could have induced bone thickening, but in 1970, human biologist Stanley Marion Garn demonstrated there is a low correlation between the two at least in modern humans. Garn instead noted different races have different average cortical bone thicknesses, and concluded it is genetic rather than environmental. It is unclear if the condition is caused by increased bone apposition (bone formation) or decreased bone resorption, but Garn noted the stenosis is quite similar to the congenital condition in modern humans induced by hyper-apposition. In 1985, biological anthropologist Gail Kennedy argued for resorption as a result of hyperparathyroidism caused by hypocalcemia (calcium deficiency), a consequence of a dietary shift to low-calcium meat. Kennedy could not explain why the calcium metabolism of H. erectus never adjusted.[115] In 1985, American paleoanthropologist Mary Doria Russell and colleagues argued the supraorbital torus is a response to withstanding major bending moment which localizes in that region when significant force is applied through the front teeth, such as while using the mouth as a third hand to carry objects.[116]
In 2004, Noel Boaz and Russel Ciochon suggested it was a result of a cultural practice, wherein H. erectus would fight each other with fists, stones, or clubs to settle disputes or battle for mates, since the skull is reinforced in key areas. The mandible is quite robust, capable of absorbing heavy blows (no "glass jaw"); the heavy brow ridge protects the eyes, and transitions into a bar covering the ears, connecting all the way in the back of the skull, meaning blows to any of these regions can be effectively dissipated across the skull; and the sagittal keel protects the top of the braincase. Many skullcaps bear usually debilitating fractures, such as the Peking Man skull X, yet they can show signs of surviving and healing. Anthropologist Peter Brown suggested a similar reason for the unusual thickening of the modern Australian Aboriginal skull, a result of a ritual popular in central and southeast Australian tribes where adversaries would wack each other with waddies (sticks) until knockout.[114]
Culture[edit]
Social structure[edit]

The only fossil evidence regarding H. erectus group composition comes from 4 sites outside of Ileret, Kenya, where 97 footprints made 1.5 Mya were likely left by a group of at least 20 individuals. One of these trackways, based on the size of the footprints, may have been an entirely male group, which could indicate they were some specialised task group, such as a hunting or foraging party, or a border patrol. If correct, this would also indicate sexual division of labour, which distinguishes human societies from those of other great apes and social mammalian carnivores. In modern hunter gatherer societies who target large prey items, typically male parties are dispatched to bring down these high-risk animals, and, due to the low success rate, female parties focus on more predictable foods.[99] Based on modern day savanna chimp and baboon group composition and behavior, H. erectus ergaster may have lived in large, multi-male groups in order to defend against large savanna predators in the open and exposed environment.[117] However, dispersal patterns indicate that H. erectus generally avoided areas with high carnivore density.[118] It is possible that male–male bonding and male–female friendships were important societal aspects.[117]
Because H. erectus children had faster brain growth rates, H. erectus likely did not exhibit the same degree of maternal investment or child-rearing behaviours as modern humans.[94]
Because H. erectus males and females are thought to have been about the same size compared to other great apes (exhibit less size-specific sexual dimorphism), it is generally hypothesised that they lived in a monogamous society, as reduced sexual dimorphism in primates is typically correlated with this mating system.[96] However, it is unclear if H. erectus did in fact exhibit humanlike rates of sexual dimorphism.[20] If they did, then it would mean only female height increased from the ancestor species, which could have been caused by a shift in female fertility or diet, and/or reduced pressure on males for large size. This in turn could imply a shift in female behavior which made it difficult for males to maintain a harem.[119]
Food[edit]
Increasing brain size is often directly associated with a meatier diet and resultant higher caloric intake. Human entomophagy and therefore an increase in protein consumption through insects has also been proposed as a possible cause. However, it is also possible that the energy-expensive guts decreased in size in H. erectus, because the large ape gut is used to synthesize fat by fermenting plant matter which was replaced by dietary animal fat, allowing more energy to be diverted to brain growth. This would have increased brain size indirectly while maintaining the same caloric requirements of ancestor species. H. erectus may have also been the first to use a hunting and gathering food collecting strategy as a response to the increasing dependence on meat. With an emphasis on teamwork, division of labor, and food sharing, hunting and gathering was a dramatically different subsistence strategy from previous modes.[88][113]

H. erectus sites frequently are associated with assemblages of medium- to large-sized game, namely elephants, rhinos, hippos, bovine, and boar. H. erectus would have had considerable leftovers, potentially pointing to food sharing or long-term food preservation (such as by drying) if most of the kill was indeed utilized. It is possible that H. erectus grew to become quite dependent on large-animal meat, and the disappearance of H. erectus from the Levant is correlated with the local extinction of the straight-tusked elephant.[113] Nonetheless, H. erectus diet likely varied widely depending upon location. For example, at the 780 kya Gesher Benot Ya'aqov site, Israel, the inhabitants gathered and ate 55 different types of fruits, vegetables, seeds, nuts, and tubers, and it appears that they used fire to roast certain plant materials that otherwise would have been inedible; they also consumed amphibians, reptiles, birds, aquatic and terrestrial invertebrates, in addition to the usual large creatures such as elephant and fallow deer.[120] At the 1.95 Mya FwJJ20 lakeside site in the East Turkana Basin, Kenya, the inhabitants ate (alongside the usual bovids, hippos, and rhinos) aquatic creatures such as turtles, crocodiles, and catfish. The large animals were likely scavenged at this site, but the turtles and fish were possibly collected live.[121] In East Africa between 2.0 and 1.4 Mya, carcasses of C4-grazing ungulates, particularly alcelaphins, featured increasingly prominently in the diet of these hominins.[122] At the 1.5 Mya Trinil H. K. site, Java, H. erectus likely gathered fish and shellfish.[123]
Dentally, H. erectus mouths were not as versatile as those of ancestor species, capable of processing a narrower range of foods. However, tools were likely used to process hard foods, thus affecting the chewing apparatus, and this combination may have instead increased dietary flexibility (though this does not equate to a highly varied diet). Such versatility may have permitted H. erectus to inhabit a range of different environments, and migrate beyond Africa.[88]
In 1999, British anthropologist Richard Wrangham proposed the "cooking hypothesis" which states that H. erectus speciated from the ancestral H. habilisbecause of fire usage and cooking 2 million years ago to explain the rapid doubling of brain size between these two species in only a 500,000 year timespan, and the sudden appearance of the typical human body plan. Cooking makes protein more easily digestible, speeds up nutrient absorption, and destroys food-borne pathogens, which would have increased the environment's natural carrying capacity, allowing group size to expand, causing selective pressure for sociality, requiring greater brain function.[124][125] However, the fossil record does not associate the emergence of H. erectus with fire usage nor with any technological breakthrough for that matter, and cooking likely did not become a common practice until after 400 kya.[88][113]
Java Man's dispersal through Southeast Asia coincides with the extirpation of the giant turtle Megalochelys, possibly due to overhunting as the turtle would have been an easy, slow-moving target which could have been stored for quite some time.[126]
Technology[edit]
Tool production[edit]
H. erectus is credited with inventing the Acheulean stone tool industry, succeeding the Oldowan industry,[127][128] and were the first to make lithic flakes bigger than 10 cm (3.9 in), and hand axes (which includes bifacial tools with only 2 sides, such as picks, knives, and cleavers).[129] Though larger and heavier, these hand axes had sharper, chiseled edges.[130] They were likely multi-purpose tools, used in variety of activities such as cutting meat, wood, or edible plants.[131] In 1979, American paleontologist Thomas Wynn stated that Acheulean technology required operational intelligence (foresight and planning), being markedly more complex than Oldowan technology which included lithics of unstandardized shape, cross-sections, and symmetry. Based on this, he concluded that there is not a significant disparity in intelligence between H. erectus and modern humans and that, for the last 300,000 years, increasing intelligence has not been a major influencer of cultural evolution.[132] However, a 1 year old H. erectus specimen shows that this species lacked an extended childhood required for greater brain development, indicating lower cognitive capabilities.[110] A few sites, likely due to occupation over several generations, features hand axes en masse, such as at Melka Kunture, Ethiopia; Olorgesailie, Kenya; Isimila, Tanzania; and Kalambo Falls, Zambia.[131]
The earliest record of Acheulean technology comes from West Turkana, Kenya 1.76 Mya. Oldowan lithics are also known from the site, and the two seemed to coexist for some time. The earliest records of Acheulean technology outside of Africa date to no older than 1 Mya, indicating it only became widespread after some secondary H. erectusdispersal from Africa.[130]
On Java, H. erectus produced tools from shells at Sangiran[133] and Trinil.[134] Spherical stones, measuring 6–12 cm (2.4–4.7 in) in diameter, are frequently found in African and Chinese Lower Paleolithic sites, and were potentially used as bolas; if correct, this would indicate string and cordage technology.[135]
Fire[edit]
H. erectus is credited as the first human ancestor to have used fire, though the timing of this invention is debated mainly because campfires very rarely and very poorly preserve over long periods of time, let alone thousands or millions of years. The earliest claimed fire sites are in Kenya, FxJj20 at Koobi Fora[136][124][137] and GnJi 1/6E in the Chemoigut Formation, as far back as 1.5 Mya,[124][137] and in South Africa, Wonderwerk Cave, 1.7 Mya.[138] The first firekeepers are thought to have simply transported to caves and maintained naturally occurring fires for extended periods of time or only sporadically when the opportunity arose. Maintaining fires would require firekeepers to have knowledge on slow-burning materials such as dung.[124] Fire becomes markedly more abundant in the wider archaeological record after 400,000–300,000 years ago, which can be explained as some advancement in fire management techniques took place at this time[124] or human ancestors only opportunistically used fire until this time.[137][139][88][113] It is possible that firestarting was invented and lost and reinvented multiple times and independently by different communities rather than being invented in one place and spreading throughout the world.[139] The earliest evidence of hearths comes from Gesher Benot Ya'aqov, Israel, over 700,000 years ago, where fire is recorded in multiple layers in an area close to water, both uncharacteristic of natural fires.[125]
Artificial lighting may have led to increased waking hours—modern humans have about a 16-hour waking period, whereas other apes are generally awake from only sunup to sundown—and these additional hours were probably used for socializing. Because of this, fire usage is probably also linked to the origin of language.[124][125] Artificial lighting may have also made sleeping on the ground instead of the trees possible by keeping terrestrial predators at bay.[125]
Migration into the frigid climate of Ice Age Europe may have only been possible because of fire, but evidence of fire usage in Europe until about 400–300,000 years ago is notably absent.[137] If these early European H. erectus did not have fire, it is largely unclear how they stayed warm, avoided predators, and prepared animal fat and meat for consumption. There was also a lower likelihood of naturally occurring fires due to lightning being less common in areas further north. It is possible that they only knew how to maintain fires in certain settings in the landscapes and prepared food some distance away from home, meaning evidence of fire and evidence of hominin activity are spaced far apart.[125] Alternatively, H. erectus may have only pushed farther north during warmer interglacial periods—thus not requiring fire, food storage, or clothing technology—[140] and their dispersal patterns indicate they generally stayed in warmer lower-to-middle latitudes.[118] It is debated if the H. e. pekinensis inhabitants of Zhoukoudian, Northern China, were capable of controlling fires as early as 770 kya to stay warm in what may have been a relatively cold climate.[141]
Construction[edit]

In 1962, a 366 cm × 427 cm × 30 cm (12 ft × 14 ft × 1 ft) circle made with volcanic rocks was discovered in Olduvai Gorge. At 61–76 cm (2–2.5 ft) intervals, rocks were piled up to 15–23 cm (6–9 in) high. British palaeoanthropologist Mary Leakey suggested the rock piles were used to support poles stuck into the ground, possibly to support a windbreak or a rough hut. Some modern day nomadic tribes build similar low-lying rock walls to build temporary shelters upon, bending upright branches as poles and using grasses or animal hide as a screen.[143] Dating to 1.75 Mya, it is the oldest claimed evidence of architecture.[144]
In Europe, evidence of constructed dwelling structures dating to or following the Holstein Interglacial (which began 424 kya) has been claimed in Bilzingsleben, Germany; Terra Amata, France; and Fermanville and Saint-Germain-des-Vaux in Normandy. The oldest evidence of a dwelling (and a campfire) in Europe comes from Přezletice, Czech Republic, 700 kya during the Cromerian Interglacial. This dwelling's base measured about 3 m × 4 m (9.8 ft × 13.1 ft) on the exterior and 3 m × 2 m (9.8 ft × 6.6 ft) on the interior, and is considered to have been a firm surface hut, probably with a vaulted roof made of thick branches or thin poles, supported by a foundation of big rocks and earth, and likely functioned as a winter base camp.[145]
The earliest evidence of cave habitation is Wonderwerk Cave, South Africa, about 1.6 Mya, but evidence of cave use globally is sporadic until about 600 kya.[146]
Clothing[edit]

It is largely unclear when clothing was invented, with the earliest estimate stretching as far back as 3 Mya to compensate for a lack of insulating body hair.[103] It is known that head lice and body lice (the latter can only inhabit clothed individuals) for modern humans diverged about 170 kya, well before modern humans left Africa, meaning clothes were already well in use before encountering cold climates. One of the first uses of animal hide is thought to have been for clothing, and the oldest hide scrapers date to about 780 kya, though this is not indicative of clothing.[147]
Seafaring[edit]
Acheulean artifacts discovered on isolated islands that were never connected to land in the Pleistocene may show seafaring by H. erectus as early as 1 Mya in Indonesia. They had arrived on the islands of Flores, Timor, and Roti, which would have necessitated crossing the Lombok Strait (the Wallace Line), at least before 800 kya. It is also possible they were the first European mariners as well and crossed the Strait of Gibraltar between North Africa and Spain. A 2021 genetic analysis of these island populations of H. erectus found no evidence of interbreeding with modern humans.[148] Seafaring capability would show H. erectus had a great capacity for planning, likely months in advance of the trip.[149][150]
Similarly, Homo luzonensis is dated between 771,000 and 631,000 years ago. Because Luzon has always been an island in the Quaternary, the ancestors of H. luzonensis would have had to have made a substantial sea crossing and crossed the Huxley Line.[151]
Healthcare[edit]

The earliest probable example of infirming sick group members is a 1.77 Mya H. e. georgicus specimen who had lost all but one tooth due to age or gum disease, the earliest example of severe chewing impairment, yet still survived for several years afterwards. However, it is possible australopithecines were capable of caring for debilitated group members.[152]Unable to chew, this H. e. georgicus individual probably ate soft plant or animal foods possibly with assistance from other group members. High-latitude groups are thought to have been predominantly carnivorous, eating soft tissue such as bone marrow or brains, which may have increased survival rates for toothless individuals.[153]
The 1.5 Mya Turkana boy was diagnosed with juvenile spinal disc herniation, and, because this specimen was still growing, this caused some scoliosis (abnormal curving of the spine). These usually cause recurrent lower back pain and sciatica (pain running down the leg), and likely restricted Turkana boy in walking, bending, and other daily activities. The specimen appears to have survived into adolescence, which evidences advanced group care.[154]
The 1,000–700 kya Java man specimen presents a noticeable osteocyte on the femur, likely Paget's disease of bone, and osteopetrosis, thickening of the bone, likely resulting from skeletal fluorosis caused by ingestion of food contaminated by fluorine-filled volcanic ash (as the specimen was found in ash-filled strata). Livestock that grazes on volcanic ash ridden fields typically die of acute intoxication within a few days or weeks.[155]
Art and rituals[edit]
An engraved Pseudodon shell DUB1006-fL with geometric markings could possibly be evidence of the earliest art-making, dating back to 546–436 kya. Art-making capabilities could be considered evidence of symbolic thinking, which is associated with modern cognition and behavior.[134][156][157][158] In 1976, American archeologist Alexander Marshack asserted that engraved lines on an ox rib, associated with Acheulean lithics, from Pech de l'Azé, France, are similar to a meander design found in modern human Upper Paleolithic cave art.[159] Three ostrich eggshell beads associated with Achuelian lithics were found in northwestern Africa, the earliest disc beads ever found, and Acheulian disc beads have also been found in France and Israel.[149] The Middle Pleistocene "Venus of Tan-Tan" and "Venus of Berekhat Ram" are postulated to been crafted by H. erectus to resemble a human form. They were mostly formed by natural weathering, but slightly modified to emphasize certain grooves to suggest hairline, limbs, and eyes.[160][161] The former has traces of pigments on the front side, possibly indicating it was colored.[160]
H. erectus was also the earliest human to have intentionally collected red-colored pigments, namely ochre, recorded as early as the Middle Pleistocene. Ochre lumps at Olduvai Gorge, Tanzania—associated with the 1.4 Ma Olduvai Hominid 9—and Ambrona, Spain—which dates to 424–374 kya—were suggested to have been struck by a hammerstone and purposefully shaped and trimmed.[162][159] At Terra Amata, France—which dates to 425–400 or 355–325 kya—red, yellow, and brown ochres were recovered in association with pole structures; ochre was probably heated to achieve such a wide color range.[162][163] As it is unclear if H. erectus could have used ochre for any practical application, ochre collection might indicate that H. erectus was the earliest human to have exhibited a sense of aesthetics and to think beyond simply survival. Later human species are postulated to have used ochre as body paint, but in the case of H. erectus, it is contested if body paint was used so early in time. Further, it is unclear if these few examples are not simply isolated incidents of ochre use, as ochre is much more prevalent in Middle and Upper Paleolithic sites attributed to Neanderthals and H. sapiens.[164][159]
In 1935, Jewish-German anthropologist Franz Weidenreich speculated that the inhabitants of the Chinese Zhoukoudian Peking Man site were members of some Lower Paleolithic Skull Cult because the skulls all showed fatal blows to the head, breaking in of the foramen magnum at the base of the skull, by-and-large lack of preserved facial aspects, an apparently consistent pattern of breaking on the mandible, and a lack of post-cranial remains (elements that are not the skull). He believed that the inhabitants were headhunters, and smashed open the skulls and ate the brains of their victims.[165][159] However, scavenging animals and natural forces such as flooding can also inflict the same kind of damage to skulls,[159] and there is not enough evidence to suggest manhunting or cannibalism.[166]
In 1999, British science writers Marek Kohn and Steven Mithen said that many hand axes exhibit no wear and were produced en masse, and concluded that these symmetrical, tear-drop shaped lithics functioned primarily as display tools so males could prove their fitness to females in some courting ritual, and were discarded afterwards.[167] However, an apparent lack of reported wearing is likely due to a lack of use-wear studies, and only a few sites yield an exorbitant sum of hand axes likely due to gradual accumulation over generations instead of mass production.[131]
Language[edit]
In 1984, the vertebral column of the 1.6 Mya adolescent Turkana boy indicated that this individual did not have properly developed respiratory muscles in order to produce speech. In 2001, American anthropologists Bruce Latimer and James Ohman concluded that Turkana boy was afflicted by skeletal dysplasia and scoliosis.[168] In 2006, American anthropologist Marc Meyer and colleagues described a 1.8 Mya H. e. georgicus specimen as having a spine within the range of variation of modern human spines, contending that Turkana boy had spinal stenosis and was thus not representative of the species. Also, because he considered H. e. georgicus ancestral to all non-African H. erectus, Meyer concluded that the respiratory muscles of all H. erectus (at least non-H. ergaster) would not have impeded vocalisation or speech production.[169] However, in 2013 and 2014, anthropologist Regula Schiess and colleagues concluded that there is no evidence of any congenital defects in Turkana boy, and considered the specimen representative of the species.[170][171]
Neurologically, all Homo have similarly configured brains, and, likewise, the Broca's and Wernicke's areas (in charge of sentence formulation and speech production in modern humans) of H. erectus were comparable to those of modern humans. However, this is not indicative of anything in terms of speech capability as even large chimpanzees can have similarly expanded Broca's area, and it is unclear if these areas served as language centers in archaic humans.[172] A 1-year-old H. erectus specimen shows that an extended childhood to allow for brain growth, which is a prerequisite in language acquisition, was not exhibited in this species.[110]
The hyoid bone supports the tongue and makes possible modulation of the vocal tract to control pitch and volume. A 400 kya H. erectus hyoid bone from Castel di Guido, Italy, is bar-shaped—more similar to that of other Homo than to that of non-human apes and Australopithecus—but is devoid of muscle impressions, has a shield-shaped body, and is implied to have had reduced greater horns, meaning H. erectus lacked a humanlike vocal apparatus and thus anatomical prerequisites for a modern human level of speech.[173] Increasing brain size and cultural complexity in tandem with technological refinement, and the hypothesis that articulate Neanderthals and modern humans may have inherited speech capabilities from the last common ancestor, could possibly indicate that H. erectus used some proto-language and built the basic framework which fully fledged languages would eventually be built around.[174] However, this ancestor may have instead been H. heidelbergensis, as a hyoid bone of a 530 kya H. heidelbergensis specimen from the Spanish Sima de los Huesos Cave is like that of modern humans,[175] and another specimen from the same area shows an auditory capacity sensitive enough to pick up human speech.[176]
Extinction[edit]
The last known occurrence of Homo erectus is 117,000–108,000 years ago in Ngandong, Java according to a study published in 2019.[1]
In 2020 researchers reported that Homo erectus and Homo heidelbergensis lost more than half of their climate niche – climate they were adapted to – with no corresponding reduction in physical range, just before extinction and that climate change played a substantial role in extinctions of past Homospecies.[177][178][179]
Fossils[edit]

The lower cave of China's Zhoukoudian Cave is one of the most important archaeological sites worldwide.[180]There have been remains of 45 Homo erectus individuals found and thousands of tools recovered.[180] Most of these remains were lost during World War 2, with the exception of two postcranial elements that were rediscovered in China in 1951 and four human teeth from 'Dragon Bone Hill'.[180]
New evidence has shown that Homo erectus does not have uniquely thick vault bones, as was previously thought.[181] Testing showed that neither Asian nor African Homo erectus had uniquely large vault bones.[181]
Individual fossils[edit]
Some of the major Homo erectus fossils:
- Indonesia (island of Java): Trinil 2 (holotype), Sangiran collection, Sambungmachan collection,[182] Ngandong collection
- China ("Peking Man"): Lantian (Gongwangling and Chenjiawo), Yunxian, Zhoukoudian, Nanjing, Hexian
- Kenya: KNM ER 3883, KNM ER 3733
- Vietnam: Northern, Tham Khuyen,[183] Hoa Binh[citation needed]
- Republic of Georgia: Dmanisi collection ("Homo erectus georgicus")
- Ethiopia: Daka calvaria
- Eritrea: Buia cranium (possibly H. ergaster)[184]
- Denizli Province, Turkey: Kocabas fossil[106]
- Drimolen, South Africa: DNH 134[185]
Phylogeny[edit]
A cladogram of Homo erectus is as follows.[186] It is indicated how many million years ago the clades diverged.
| Homo (2.85) |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Homo erectus was originally African. The extant Homo heidelbergensis (cladistically granting Homo sapiens), which was originally African, emerged within the Asian Homo erectus. Contemporary groups appear to have been interbreeding, so any phylogeny like this only gives a coarse impression of the evolution of Homo, and extinct lineage may have partially continued in other groupings. Not included are other contemporary groups such as Homo floresiensis, Homo naledi, Homo luzonensis, Homo rudolfensis, Australopithecus sediba, Australopithecus africanus, and Paranthropus.
Gallery[edit]
Giant squid
| Giant squid | |
|---|---|
 | |
| Giant squid, Architeuthis sp., modified from an illustration by A. E. Verrill, 1880 | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Mollusca |
| Class: | Cephalopoda |
| Order: | Oegopsida |
| Superfamily: | Architeuthoidea |
| Family: | Architeuthidae Pfeffer, 1900 |
| Genus: | Architeuthis Steenstrup in Harting, 1860 |
| Species: | A. dux |
| Binomial name | |
| Architeuthis dux Steenstrup, 1857 | |
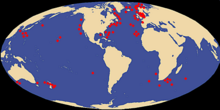 | |
| Worldwide giant squid distribution based on recovered specimens | |
| Synonyms | |
| |
The giant squid (Architeuthis dux) is a species of deep-ocean dwelling squid in the family Architeuthidae. It can grow to a tremendous size, offering an example of abyssal gigantism: recent estimates put the maximum size at around 12–13 m (39–43 ft)[2][3][4][5] for females and 10 m (33 ft)[3] for males, from the posterior fins to the tip of the two long tentacles (longer than the colossal squid at an estimated 9–10 m (30–33 ft),[6] but substantially lighter, as the tentacles make up most of the length[7]). The mantle of the giant squid is about 2 m (6 ft 7 in) long (more for females, less for males), and the length of the squid excluding its tentacles (but including head and arms) rarely exceeds 5 m (16 ft).[3] Claims of specimens measuring 20 m (66 ft) or more have not been scientifically documented.[3]
The number of different giant squid species has been debated, but genetic research suggests that only one species exists.[8]
The first images of the animal in its natural habitat were taken in 2004 by a Japanese team.[9]
Taxonomy[edit]
The closest relatives of the giant squid are thought to be the four obscure species of "neosquid" in the family Neoteuthidae, each of which belongs to its own monotypic genus, as with the giant squid. Together, both families comprise the superfamily Architeuthoidea.[10]
Range and habitat[edit]
The giant squid is widespread, occurring in all of the world's oceans. It is usually found near continental and island slopes from the North Atlantic Ocean, especially Newfoundland, Norway, the northern British Isles, Spain and the oceanic islands of the Azores and Madeira, to the South Atlantic around southern Africa, the North Pacific around Japan, and the southwestern Pacific around New Zealand and Australia.[11] Specimens are rare in tropical and polar latitudes.
The vertical distribution of giant squid is incompletely known, but data from trawled specimens and sperm whale diving behavior suggest it spans a large range of depths, possibly 300–1,000 metres (980–3,280 ft).[12]
Morphology and anatomy[edit]
This section needs additional citations for verification. (September 2020) |
Like all squid, a giant squid has a mantle (torso), eight arms, and two longer tentacles (the longest known tentacles of any cephalopod). The arms and tentacles account for much of the squid's great length, making it much lighter than its chief predator, the sperm whale. Scientifically documented specimens have masses of hundreds, rather than thousands, of kilograms.[citation needed]

The inside surfaces of the arms and tentacles are lined with hundreds of subspherical suction cups, 2 to 5 cm (0.79 to 1.97 in) in diameter, each mounted on a stalk. The circumference of these suckers is lined with sharp, finely serrated rings of chitin.[13] The perforation of these teeth and the suction of the cups serve to attach the squid to its prey. It is common to find circular scars from the suckers on or close to the head of sperm whales that have attacked giant squid.[citation needed]
Each tentacular club is divided into three regions—the carpus ("wrist"), manus ("hand") and dactylus("finger").[14][15] The carpus has a dense cluster of cups, in six or seven irregular, transverse rows. The manus is broader, closer to the end of the club, and has enlarged suckers in two medial rows. The dactylus is the tip. The bases of all the arms and tentacles are arranged in a circle surrounding the animal's single, parrot-like beak, as in other cephalopods.[citation needed]
Giant squid have small fins at the rear of their mantles used for locomotion. Like other cephalopods, they are propelled by jet—by pulling water into the mantle cavity, and pushing it through the siphon, in gentle, rhythmic pulses. They can also move quickly by expanding the cavity to fill it with water, then contracting muscles to jet water through the siphon. Giant squid breathe using two large gills inside the mantle cavity. The circulatory system is closed, which is a distinct characteristic of cephalopods. Like other squid, they contain dark ink.[16]

The giant squid has a sophisticated nervous system and complex brain, attracting great interest from scientists. It also has the largest eyes of any living creature except perhaps the colossal squid—up to at least 27 cm (11 in) in diameter, with a 9 cm (3.5 in) pupil (only the extinct ichthyosaurs are known to have had larger eyes).[17] Large eyes can better detect light (including bioluminescent light), which is scarce in deep water. The giant squid probably cannot see color, but it can probably discern small differences in tone, which is important in the low-light conditions of the deep ocean.[18]
Giant squid and some other large squid species maintain neutral buoyancy in seawater through an ammonium chloridesolution which is found throughout their bodies and is lighter than seawater. This differs from the method of flotation used by most fish, which involves a gas-filled swim bladder. The solution tastes somewhat like salty liquorice/salmiak and makes giant squid unattractive for general human consumption.[citation needed]
Like all cephalopods, giant squid use organs called statocysts to sense their orientation and motion in water. The age of a giant squid can be determined by "growth rings" in the statocyst's statolith, similar to determining the age of a tree by counting its rings. Much of what is known about giant squid age is based on estimates of the growth rings and from undigested beaks found in the stomachs of sperm whales.[citation needed]
Size[edit]
The giant squid is the second-largest mollusc and one of the largest of all extant invertebrates. It is exceeded only by the colossal squid, Mesonychoteuthis hamiltoni, which may have a mantle nearly twice as long. Several extinct cephalopods, such as the Cretaceous coleoid Yezoteuthis and Haboroteuthis,[19][20] and the Ordovician nautiloid Endoceras[21] may have grown even larger. Although the Cretaceous Tusoteuthis, with its 2 m (6 ft 6.7 in) long mantle, was once considered to grow to a size close to that of the giant squid (over 10 m (32.8 ft) including arms), this genus is likely doubtful. The largest specimen probably belonged to the genus Enchoteuthis, estimated to have short arms, with a total length of only 3 m (9.8 ft).[22]
Giant squid size, particularly total length, has often been exaggerated. Reports of specimens reaching and even exceeding 20 m (66 ft) are widespread, but no specimens approaching this size have been scientifically documented.[3] According to giant squid expert Steve O'Shea, such lengths were likely achieved by greatly stretching the two tentacles like elastic bands.[3]

Based on the examination of 130 specimens and of beaks found inside sperm whales, giant squids' mantles are not known to exceed 2.25 m (7 ft 4.6 in).[3][4]Including the head and arms, but excluding the tentacles, the length very rarely exceeds 5 m (16 ft).[3] Maximum total length, when measured relaxed post mortem, is estimated at 12 m (39 ft) or 13 m (43 ft) for females and 10 m (33 ft) for males from the posterior fins to the tip of the two long tentacles.[2][3][4][5]
Giant squid exhibit sexual dimorphism. Maximum weight is estimated at 275 kg (606 lb) for females and 150 kg (330 lb) for males.[3]
Reproductive cycle[edit]
Little is known about the reproductive cycle of giant squid. They are thought to reach sexual maturity at about three years old; males reach sexual maturity at a smaller size than females. Females produce large quantities of eggs, sometimes more than 5 kg (11 lb), that average 0.5 to 1.4 mm (0.020 to 0.055 in) long and 0.3 to 0.7 mm (0.012 to 0.028 in) wide.[23] Females have a single median ovary in the rear end of the mantle cavity and paired, convoluted oviducts, where mature eggs pass exiting through the oviducal glands, then through the nidamental glands. As in other squid, these glands produce a gelatinous material used to keep the eggs together once they are laid.[24]
In males, as with most other cephalopods, the single, posterior testis produces sperm that move into a complex system of glands that manufacture the spermatophores. These are stored in the elongate sac, or Needham's sac, that terminates in the penis from which they are expelled during mating. The penis is prehensile, over 90 cm (35 in) long, and extends from inside the mantle. The two ventral arms on a male giant squid are hectocotylized, which means they are specialized to facilitate the fertilization of the female's eggs.[25]
How the sperm is transferred to the egg mass is much debated, as giant squid lack the hectocotylus used for reproduction in many other cephalopods. It may be transferred in sacs of spermatophores, called spermatangia, which the male injects into the female's arms.[26] This is suggested by a female specimen recently found in Tasmania,[27] having a small subsidiary tendril attached to the base of each arm.
Post-larval juveniles have been discovered in surface waters off New Zealand, with plans to capture more and maintain them in an aquarium to learn more about the creature.[28] Young giant squid specimens were found off the coast of southern Japan in 2013 and confirmed through genetic analysis.[29]
Another juvenile, approximately 3.7 metres long, was encountered and filmed alive in the harbour in the Japanese city of Toyama on 24 December 2015; after being filmed and viewed by a large number of spectators, including a diver who entered the water to film the squid up close, it was guided out of the harbour into Toyama Bay by the diver.[30][31]
Genetics[edit]
Analysis of the mitochondrial DNA of giant squid individuals from all over the world has found that there is little variation between individuals across the globe (just 181 differing genetic base pairs out of 20,331). This suggests that there is only a single species of giant squid in the world. Squid larvae may be dispersed by ocean currents across vast distances.[8]
Ecology[edit]
Feeding[edit]

Recent studies have shown giant squid feed on deep-sea fish, such as the orange roughy (Hoplostethus atlanticus), and other squid species.[32][33] They catch prey using the two tentacles, gripping it with serrated sucker rings on the ends. Then they bring it toward the powerful beak, and shred it with the radula (tongue with small, file-like teeth) before it reaches the esophagus. They are believed to be solitary hunters, as only individual giant squid have been caught in fishing nets. Although the majority of giant squid caught by trawl in New Zealand waters have been associated with the local hoki (Macruronus novaezelandiae) fishery, hoki do not feature in the squid's diet. This suggests giant squid and hoki prey on the same animals.[32]
Predators and potential cannibalism[edit]
The known predators of adult giant squid include sperm whales, pilot whales,[34][35] southern sleeper sharks,[36]and in some regions killer whales.[37] Juveniles may fall prey to other large deep sea predators. Because sperm whales are skilled at locating giant squid, scientists have tried to observe them to study the squid. Giant squid have also been recently discovered to presumably steal food from each other;[38] in mid-to-late October 2016, a 9 m (30 ft) giant squid washed ashore in Galicia, Spain. The squid had been photographed alive shortly before its death by a tourist named Javier Ondicol, and examination of its corpse by the Coordinators for the Study and Protection of Marine Species (CEPESMA) indicates that the squid was attacked and mortally wounded by another giant squid, losing parts of its fins, and receiving damage to its mantle, one of its gills and losing an eye. The intact nature of the specimen indicates that the giant squid managed to escape its rival by slowly retreating to shallow water, where it died of its wounds. The incident is the second to be documented among Architeuthis recorded in Spain, with the other occurring in Villaviciosa. Evidence in the form of giant squid stomach contents containing beak fragments from other giant squid in Tasmania also supports the theory that the species is at least occasionally cannibalistic. Alternatively, such squid-on-squid attacks may be a result of competition for prey. These traits are seen in the Humboldt squid as well, indicating that cannibalism in large squid may be more common than originally thought.[39]
Population[edit]
Scientists have been unable to determine the worldwide population of giant squid to any degree of accuracy. Estimates have been put together based on the number of giant squid beaks found in the stomachs of deceased sperm whales, a known predator of the giant squid, and the better known population of sperm whales. Based on such observations, it has been estimated that sperm whales consume between 4.3 and 131 million giant squid annually, implying that the giant squid population is likewise well into the millions, but more precise estimates have been elusive.[40]
Species[edit]

The taxonomy of the giant squid, as with many cephalopod genera, has long been debated. Lumpers and splitters may propose as many as seventeen species or as few as one. The broadest list is:[citation needed]
- Architeuthis dux, Atlantic giant squid
- Architeuthis (Loligo) hartingii
- Architeuthis japonica
- Architeuthis kirkii
- Architeuthis (Megateuthis) martensii, North Pacific giant squid
- Architeuthis physeteris
- Architeuthis sanctipauli, southern giant squid
- Architeuthis (Steenstrupia) stockii
- Architeuthis (Loligo) bouyeri
- Architeuthis clarkei
- Architeuthis (Plectoteuthis) grandis
- Architeuthis (Megaloteuthis) harveyi
- Architeuthis longimanus
- Architeuthis monachus?
- Architeuthis nawaji
- Architeuthis princeps
- Architeuthis (Dubioteuthis) physeteris
- Architeuthis titan
- Architeuthis verrilli
It is unclear if these are distinct species, as no genetic or physical basis for distinguishing between them has yet been proposed.
In the 1984 FAO Species Catalogue of the Cephalopods of the World, Roper, et al. wrote:[41]
In Cephalopods: A World Guide (2000), Mark Norman writes:[42]
In March 2013, researchers at the University of Copenhagen suggested that, based on DNA research, there is only one species:[8][43]
Timeline[edit]
This section needs additional citations for verification. (September 2020) |

Aristotle, who lived in the fourth century BC, described a large squid, which he called teuthus, distinguishing it from the smaller squid, the teuthis. He mentions, "of the calamaries, the so-called teuthus is much bigger than the teuthis; for teuthi [plural of teuthus] have been found as much as five ells long".[44]
Pliny the Elder, living in the first century AD, also described a gigantic squid in his Natural History, with the head "as big as a cask", arms 30 ft (9.1 m) long, and carcass weighing 700 lb (320 kg).[45]: 11 [46][47]
Tales of giant squid have been common among mariners since ancient times, and may have led to the Norse legend of the kraken,[48] a tentacled sea monster as large as an island capable of engulfing and sinking any ship. Japetus Steenstrup, the describer of Architeuthis, suggested a giant squid was the species described as a sea monk to the Danish king Christian III circa 1550.[49] The Lusca of the Caribbean and Scylla in Greek mythology may also derive from giant squid sightings. Eyewitness accounts of other sea monsters like the sea serpent are also thought[by whom?] to be mistaken interpretations of giant squid.[citation needed]
Steenstrup wrote a number of papers on giant squid in the 1850s. He first used the term "Architeuthus" (this was the spelling he chose) in a paper in 1857. A portion of a giant squid was secured by the French corvette Alecton in 1861, leading to wider recognition of the genus in the scientific community. From 1870 to 1880, many squid were stranded on the shores of Newfoundland. For example, a specimen washed ashore in Thimble Tickle Bay, Newfoundland, on 2 November 1878; its mantle was reported to be 6.1 m (20 ft) long, with one tentacle 10.7 m (35 ft) long, and it was estimated as weighing 1 short ton (0.9 t).[50] Many of these specimens were not preserved, often being processed into manure or animal feed.[50] In 1873, a squid "attacked" a minister and a young boy in a dory near Bell Island, Newfoundland. Many strandings also occurred in New Zealand during the late 19th century.[citation needed]

Although strandings continue to occur sporadically throughout the world, none have been as frequent as those at Newfoundland and New Zealand in the 19th century. It is not known why giant squid become stranded on shore, but it may be because the distribution of deep, cold water where squid live is temporarily altered. Many scientists who have studied squid mass strandings believe they are cyclical and predictable. The length of time between strandings is not known, but was proposed to be 90 years by Architeuthis specialist Frederick Aldrich. Aldrich used this value to correctly predict a relatively small stranding that occurred between 1961 and 1968.[45]
In 2004, another giant squid, later named "Archie", was caught off the coast of the Falkland Islands by a fishing trawler. It was 8.62 m (28.3 ft) long and was sent to the Natural History Museum in London to be studied and preserved. It was put on display on 1 March 2006 at the Darwin Centre.[51][52][53] The find of such a large, complete specimen is very rare, as most specimens are in a poor condition, having washed up dead on beaches or been retrieved from the stomachs of dead sperm whales.
Researchers undertook a painstaking process to preserve the body. It was transported to England on ice aboard the trawler; then it was defrosted, which took about four days. The major difficulty was that thawing the thick mantle took much longer than the tentacles. To prevent the tentacles from rotting, scientists covered them in ice packs, and bathed the mantle in water. Then they injected the squid with a formol-saline solution to prevent rotting. The creature is now on show in a 9 m (30 ft) glass tank at the Darwin Centre of the Natural History Museum.[citation needed]


In December 2005, the Melbourne Aquarium in Australia paid A$100,000 for the intact body of a 7-metre-long (23 ft) giant squid, preserved in a giant block of ice, which had been caught by fishermen off the coast of New Zealand's South Island that year.[52]
The number of known giant squid specimens was close to 700 in 2011,[54] and new ones are reported each year. Around 30 of these specimens are exhibited at museums and aquaria worldwide.[54] The Museo del Calamar Gigante in Luarca, Spain, had by far the largest collection on public display, but many of the museum's specimens were destroyed during a storm in February 2014.[55]
The search for a live Architeuthis specimen includes attempts to find live young, including larvae. The larvae closely resemble those of Nototodarus and Onykia, but are distinguished by the shape of the mantle attachment to the head, the tentacle suckers, and the beaks.[citation needed]
Images and video of live animals[edit]
By the turn of the 21st century, the giant squid remained one of the few extant megafauna to have never been photographed alive, either in the wild or in captivity. Marine biologist and author Richard Ellis described it as "the most elusive image in natural history".[56][45]: 211 In 1993, an image purporting to show a diver with a live giant squid (identified as Architeuthis dux) was published in the book European Seashells.[57] However, the animal in this photograph was a sick or dying Onykia robusta, not a giant squid.[42][45]: 211 The first footage of live (larval) giant squid ever captured on film was in 2001. The footage was shown on Chasing Giants: On the Trail of the Giant Squid on the Discovery Channel.[58]
First images of live adult[edit]

The first image of a live mature giant squid was taken on 15 January 2002, on Goshiki beach, Amino Cho, Kyoto Prefecture, Japan.[60][61][62][63][64] The animal, which measured about 2 m (6 ft 7 in) in mantle length and 4 m (13 ft) in total length,[61] was found near the water's surface. It was captured and tied to a quay, where it died overnight.[61] The specimen was identified by Koutarou Tsuchiya of the Tokyo University of Fisheries. It is on display at the National Science Museum of Japan.
First observations in the wild[edit]
The first photographs of a live giant squid in its natural habitat were taken on 30 September 2004, by Tsunemi Kubodera (National Science Museum of Japan) and Kyoichi Mori (Ogasawara Whale Watching Association).[9]Their teams had worked together for nearly two years to accomplish this. They used a five-ton fishing boat and only two crew members. The images were created on their third trip to a known sperm whale hunting ground 970 km (600 mi) south of Tokyo, where they had dropped a 900 m (3,000 ft) line baited with squid and shrimp. The line also held a camera and a flash. After over twenty tries that day, an 8 m (26 ft) giant squid attacked the lure and snagged its tentacle. The camera took over 500 photos before the squid managed to break free after four hours. The squid's 5.5 m (18 ft) tentacle remained attached to the lure. Later DNA tests confirmed the animal as a giant squid.[9]
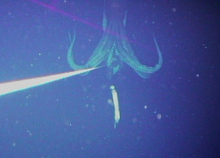
On 27 September 2005, Kubodera and Mori released the photographs to the world. The photo sequence, taken at a depth of 900 metres (3,000 ft) off Japan's Ogasawara Islands, shows the squid homing in on the baited line and enveloping it in "a ball of tentacles". The researchers were able to locate the likely general location of giant squid by closely tailing the movements of sperm whales. According to Kubodera, "we knew that they fed on the squid, and we knew when and how deep they dived, so we used them to lead us to the squid". Kubodera and Mori reported their observations in the journal Proceedings of the Royal Society.[9]
Among other things, the observations demonstrate actual hunting behaviors of adult Architeuthis, a subject on which there had been much speculation. The photographs showed an aggressive hunting pattern by the baited squid, leading to it impaling a tentacle on the bait ball's hooks. This may disprove the theory that the giant squid is a drifter which eats whatever floats by, rarely moving so as to conserve energy. It seems the species has a much more aggressive feeding technique.
First video of live adult[edit]
In November 2006, American explorer and diver Scott Cassell led an expedition to the Gulf of California with the aim of filming a giant squid in its natural habitat. The team employed a novel filming method: using a Humboldt squid carrying a specially designed camera clipped to its fin. The camera-bearing squid caught on film what was claimed to be a giant squid, with an estimated length of 40 feet (12 m), engaging in predatory behavior.[65][66] The footage aired a year later on a History Channel program, MonsterQuest: Giant Squid Found.[66] Cassell subsequently distanced himself from this documentary, claiming that it contained multiple factual and scientific errors.[67] Videos of live giant squids have been captured three times subsequently, with one of these aforementioned individuals being guided back into the open ocean after appearing in Toyama Harbor on December 24, 2015.[68][69][70][71][72][73][74]
Second video of giant squid in natural habitat[edit]
On 19 June 2019, in an expedition run by the National Oceanic & Atmospheric Association (NOAA),[75] known as the Journey to Midnight, biologists Nathan J. Robinson and Edith Widder captured a video of a juvenile giant squid at a depth of 759 meters (2,490 feet) in the Gulf of Mexico. Michael Vecchione, a NOAA Fisheries zoologist, confirmed that the captured footage was that of the genus Architeuthis, and that the individual filmed measured at somewhere between 10 and 12 ft (3.0 and 3.7 m).
Cultural depictions[edit]

The elusive nature of the giant squid and its foreign appearance, often perceived as terrifying, have firmly established its place in the human imagination. Representations of the giant squid have been known from early legends of the krakenthrough books such as Moby-Dick and Twenty Thousand Leagues Under the Sea on to novels such as Ian Fleming's Dr. No, Peter Benchley's Beast (adapted as a film called The Beast), and Michael Crichton's Sphere (adapted as a film), and modern animated television programs.
In particular, the image of a giant squid locked in battle with a sperm whale is a common one, although the squid is the whale's prey and not an equal combatant.[76][failed verification]
See also[edit]
- Colossal squid, the largest squid species by mass
- Enteroctopus, a genus whose members are commonly known as giant octopuses
- Giant Squid Interpretation Site, a small museum in Glovers Harbour, Newfoundland
- Gigantic octopus, a hypothesised species of octopus
- Humboldt squid, a large species of squid and the only member of the genus Dosidicus
- Largest organisms
- Taningia danae, a large squid species of the genus Taningia






















No comments:
Post a Comment