The basis of any thesis is to bearing of truth versus compass to equal what is impossible at theory to craft theorem as the measure in an idea of what can be put forth as reality. To discussion of the quote unquote ongoing battle of The Bay Area and so many people fighting downtown through demonstrations in front of City Hall on 'Global Warming' I put to task fact.
1.) The Ice Age
2.) First melt
3.) Global warming
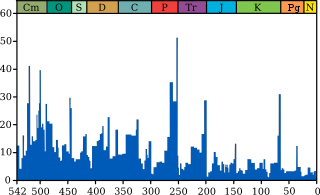
The global warming of this era is reported to have begun in an event of the dinosaur extinction over 66 million years ago (https://en.m.wikipedia.org/wiki/Cretaceous%E2%80%93Paleogene_extinction_event (https://news.nationalgeographic.com/news/2010/03/100304-snowball-earth-ice-global-warming/)) as that would have been the start and the melt and the rising temperature would have been known as global warming, recordings are in the sediment around the world. The reported current facts to date the melt are available via the latest “intel” provided at any environmental agency or documents, books of that genre leading to much more information bringing dates and dirt to ice and sand however to understand that the meteor that brought the dinosaur to its brink also creating the quote unquote Ice Age would be the beginning of understanding that upon the first melt of said event horizon or specific incident thereof would have been what is known today as "Global warming".
The point to being is that the constant blame suffered around the world or just here in The Bay Area is for not, the reality of existence (the extinction of the dinosaur) would have lead or should have brought preparatory laboratories to basis. The constant argument, the blame factor leads to only finger pointing and calculation of war, civil or not as the outcome is to grout the tile for permanent shingle saying nothing more than or less than who is what and where is when to include a bunch of quote told you so. This barrier to reality must be recognized for the thorough reality of longevity of such movements that have repeatedly laid deception, greed and so much more that it is not only the "Ice Age" that would bury Mankind now the ocean, the tides themselves will break it apart. The core to title of this paper would not jostle more as it is almost at the quoted phrase or question often repeated, "Which came first, the chicken or the egg?"
In addendum the on-going tack of the number three allows a simple introduction of picture to touch the idea of galaxy and mass to land and life as our Earth is three rocks from our Sun. To allowance of religions all over the world is to change the idea and create reality as basis from. From? Now introduce yourself to NASA, our Moon and Mars and of course and not to forget the famous Orion belt with the iconic Egyptian recognition found on Wikipedia (https://en.wikipedia.org/wiki/Orion_correlation_theory) or any other corresponding book at your local library and all of a sudden boom, the live becomes a number that can recognize that there has been instructive material that has been unfortunately overlooked. Mankind has the ability to prowess these points right down to the last decimals and yet due to the battle of wills the trust of information has been left to shows or platforms that only perpetrate fear with alien invaders (as shown on Ancient Aliens series on the History Channel (https://www.history.com/shows/ancient-aliens) or listened to on AM Radio with Coast to Coast with George Noory (https://www.coasttocoastam.com/)) or gods (Religion).
These few and simple perfections must increase the number three to more than a fixture.
Cretaceous–Paleogene extinction event

An artist's rendering of an asteroid
a few kilometers across colliding with the Earth. Such an impact can
release the equivalent energy of several million nuclear weapons
detonating simultaneously.

Complex Cretaceous–Paleogene clay layer (gray) in the Geulhemmergroeve tunnels near Geulhem, The Netherlands. (Finger is below the actual Cretaceous-Paleogene boundary)
In the geologic record, the K–Pg event is marked by a thin layer of sediment called the K–Pg boundary, which can be found throughout the world in marine and terrestrial rocks. The boundary clay shows high levels of the metal iridium, which is rare in the Earth's crust, but abundant in asteroids.[6]
As originally proposed in 1980 by a team of scientists led by Luis Alvarez and his son Walter Alvarez, it is now generally thought that the K–Pg extinction was caused by the impact of a massive comet or asteroid 10 to 15 km (6 to 9 mi) wide,[7][8] 66 million years ago,[3] which devastated the global environment, mainly through a lingering impact winter which halted photosynthesis in plants and plankton.[9][10] The impact hypothesis, also known as the Alvarez hypothesis, was bolstered by the discovery of the 180-kilometer-wide (112 mi) Chicxulub crater in the Gulf of Mexico's Yucatán Peninsula in the early 1990s,[11] which provided conclusive evidence that the K–Pg boundary clay represented debris from an asteroid impact.[12] The fact that the extinctions occurred simultaneously provides strong evidence that they were caused by the asteroid.[12] A 2016 drilling project into the Chicxulub peak ring, confirmed that the peak ring comprised granite ejected within minutes from deep in the earth, but contained hardly any gypsum, the usual sulfate-containing sea floor rock in the region: the gypsum would have vaporized and dispersed as an aerosol into the atmosphere, causing longer-term effects on the climate and food chain.
Other causal or contributing factors to the extinction may have been the Deccan Traps and other volcanic eruptions,[13][14] climate change, and sea level change.
A wide range of species perished in the K–Pg extinction, the best-known being the non-avian dinosaurs. It also destroyed a plethora of other terrestrial organisms, including certain mammals, pterosaurs, birds,[15] lizards,[16] insects,[17][18] and plants.[19] In the oceans, the K–Pg extinction killed off plesiosaurs and the giant marine lizards (Mosasauridae) and devastated fish,[20] sharks, mollusks (especially ammonites, which became extinct), and many species of plankton. It is estimated that 75% or more of all species on Earth vanished.[21] Yet the extinction also provided evolutionary opportunities: in its wake, many groups underwent remarkable adaptive radiation—sudden and prolific divergence into new forms and species within the disrupted and emptied ecological niches. Mammals in particular diversified in the Paleogene,[22] evolving new forms such as horses, whales, bats, and primates. Birds,[23] fish,[24] and perhaps lizards[16] also radiated.
Contents
Extinction patternsEdit
The event appears to have affected all continents at the same time. Non-avian dinosaurs, for example, are known from the Maastrichtian of North America, Europe, Asia, Africa, South America, and Antarctica,[25] but are unknown from the Cenozoic anywhere in the world. Similarly, fossil pollen shows devastation of the plant communities in areas as far apart as New Mexico, Alaska, China, and New Zealand.[19]
Despite the event's severity, there was significant variability in the rate of extinction between and within different clades. Species that depended on photosynthesis declined or became extinct as atmospheric particles blocked sunlight and reduced the solar energy reaching the ground. This plant extinction caused a major reshuffling of the dominant plant groups.[26] Omnivores, insectivores, and carrion-eaters survived the extinction event, perhaps because of the increased availability of their food sources. No purely herbivorous or carnivorous mammals seem to have survived. Rather, the surviving mammals and birds fed on insects, worms, and snails, which in turn fed on detritus (dead plant and animal matter).[27][28][29]
In stream communities, few animal groups became extinct, because such communities rely less directly on food from living plants, and more on detritus washed in from the land, protecting them from extinction.[30] Similar, but more complex patterns have been found in the oceans. Extinction was more severe among animals living in the water column than among animals living on or in the sea floor. Animals in the water column are almost entirely dependent on primary production from living phytoplankton, while animals on the ocean floor always or sometimes feed on detritus.[27] Coccolithophorids and mollusks (including ammonites, rudists, freshwater snails, and mussels), and those organisms whose food chain included these shell builders, became extinct or suffered heavy losses. For example, it is thought that ammonites were the principal food of mosasaurs, a group of giant marine reptiles that became extinct at the boundary.[31] The largest air-breathing survivors of the event, crocodyliforms and champsosaurs, were semi-aquatic and had access to detritus. Modern crocodilians can live as scavengers and survive for months without food, and their young are small, grow slowly, and feed largely on invertebrates and dead organisms for their first few years. These characteristics have been linked to crocodilian survival at the end of the Cretaceous.[28]
After the K–Pg extinction event, biodiversity required substantial time to recover, despite the existence of abundant vacant ecological niches.[27]
MicrobiotaEdit
The K–Pg boundary represents one of the most dramatic turnovers in the fossil record for various calcareous nanoplankton that formed the calcium deposits for which the Cretaceous is named. The turnover in this group is clearly marked at the species level.[32][33] Statistical analysis of marine losses at this time suggests that the decrease in diversity was caused more by a sharp increase in extinctions than by a decrease in speciation.[34] The K–Pg boundary record of dinoflagellates is not so well understood, mainly because only microbial cysts provide a fossil record, and not all dinoflagellate species have cyst-forming stages, which likely causes diversity to be underestimated.[27] Recent studies indicate that there were no major shifts in dinoflagellates through the boundary layer.[35]Radiolaria have left a geological record since at least the Ordovician times, and their mineral fossil skeletons can be tracked across the K–Pg boundary. There is no evidence of mass extinction of these organisms, and there is support for high productivity of these species in southern high latitudes as a result of cooling temperatures in the early Paleocene.[27] Approximately 46% of diatom species survived the transition from the Cretaceous to the Upper Paleocene, a significant turnover in species but not a catastrophic extinction.[27][36]
The occurrence of planktonic foraminifera across the K–Pg boundary has been studied since the 1930s.[37] Research spurred by the possibility of an impact event at the K–Pg boundary resulted in numerous publications detailing planktonic foraminiferal extinction at the boundary;[27] however, there is ongoing debate between groups that think the evidence indicates substantial extinction of these species at the K–Pg boundary,[38] and those who think the evidence supports multiple extinctions and expansions through the boundary.[39][40]
Numerous species of benthic foraminifera became extinct during the event, presumably because they depend on organic debris for nutrients, while biomass in the ocean is thought to have decreased. As the marine microbiota recovered, however, it is thought that increased speciation of benthic foraminifera resulted from the increase in food sources.[27] Phytoplankton recovery in the early Paleocene provided the food source to support large benthic foraminiferal assemblages, which are mainly detritus-feeding. Ultimate recovery of the benthic populations occurred over several stages lasting several hundred thousand years into the early Paleocene.[41][42]
Marine invertebratesEdit

Discoscaphites iris ammonite from the Owl Creek Formation (Upper Cretaceous), Owl Creek, Ripley, Mississippi
Ostracods, a class of small crustaceans that were prevalent in the upper Maastrichtian, left fossil deposits in a variety of locations. A review of these fossils shows that ostracod diversity was lower in the Paleocene than any other time in the Cenozoic. Current research cannot ascertain, however, whether the extinctions occurred prior to, or during, the boundary interval.[43][44]
Approximately 60% of late-Cretaceous Scleractinia coral genera failed to cross the K–Pg boundary into the Paleocene. Further analysis of the coral extinctions shows that approximately 98% of colonial species, ones that inhabit warm, shallow tropical waters, became extinct. The solitary corals, which generally do not form reefs and inhabit colder and deeper (below the photic zone) areas of the ocean were less impacted by the K–Pg boundary. Colonial coral species rely upon symbiosis with photosynthetic algae, which collapsed due to the events surrounding the K–Pg boundary;[45][46] however, the use of data from coral fossils to support K–Pg extinction and subsequent Paleocene recovery, must be weighed against the changes that occurred in coral ecosystems through the K–Pg boundary.[27]
The numbers of cephalopod, echinoderm, and bivalve genera exhibited significant diminution after the K–Pg boundary.[27] Most species of brachiopods, a small phylum of marine invertebrates, survived the K–Pg extinction event and diversified during the early Paleocene.

Rudist bivalves from the Late Cretaceous of the Omani Mountains, United Arab Emirates. Scale bar is 10 mm
Approximately 35% of echinoderm genera became extinct at the K–Pg boundary, although taxa that thrived in low-latitude, shallow-water environments during the late Cretaceous had the highest extinction rate. Mid-latitude, deep-water echinoderms were much less affected at the K–Pg boundary. The pattern of extinction points to habitat loss, specifically the drowning of carbonate platforms, the shallow-water reefs in existence at that time, by the extinction event.[49]
Other invertebrate groups, including rudists (reef-building clams) and inoceramids (giant relatives of modern scallops), also became extinct at the K–Pg boundary.[50][51]
FishEdit
There are substantial fossil records of jawed fishes across the K–Pg boundary, which provide good evidence of extinction patterns of these classes of marine vertebrates. While the deep sea realm was able to remain seemingly unaffected, there was an equal loss between the open marine apex predators and the durophagous demersal feeders on the continental shelf.Within cartilaginous fish, approximately 7 out of the 41 families of neoselachians (modern sharks, skates, and rays) disappeared after this event and batoids (skates and rays) lost nearly all the identifiable species, while more than 90% of teleost fish (bony fish) families survived.[52][53]
In the Maastrichtian age, 28 shark families and 13 batoid families thrived, of which 25 and 9, respectively, survived the K–T boundary event. Forty-seven of all neoselachian genera cross the K–T boundary, with 85% being sharks. Batoids display with 15% a comparably low survival rate.[52][54]
There is evidence of a mass extinction of bony fishes at a fossil site immediately above the K–Pg boundary layer on Seymour Island near Antarctica, apparently precipitated by the K–Pg extinction event;[55] however, the marine and freshwater environments of fishes mitigated environmental effects of the extinction event.[56]
Terrestrial invertebratesEdit
Insect damage to the fossilized leaves of flowering plants from fourteen sites in North America was used as a proxy for insect diversity across the K–Pg boundary and analyzed to determine the rate of extinction. Researchers found that Cretaceous sites, prior to the extinction event, had rich plant and insect-feeding diversity. During the early Paleocene, however, flora were relatively diverse with little predation from insects, even 1.7 million years after the extinction event.[57][58]Terrestrial plantsEdit
There is overwhelming evidence of global disruption of plant communities at the K–Pg boundary.[19][19][59][60] Extinctions are seen both in studies of fossil pollen, and fossil leaves.[19] In North America, the data suggests massive devastation and mass extinction of plants at the K–Pg boundary sections, although there were substantial megafloral changes before the boundary.[19][61] In North America, approximately 57% of plant species became extinct. In high southern hemisphere latitudes, such as New Zealand and Antarctica, the mass die-off of flora caused no significant turnover in species, but dramatic and short-term changes in the relative abundance of plant groups.[57][62] In some regions, the Paleocene recovery of plants began with recolonizations by fern species, represented as a fern spike in the geologic record; this same pattern of fern recolonization was observed after the 1980 Mount St. Helens eruption.[63]Due to the wholesale destruction of plants at the K–Pg boundary, there was a proliferation of saprotrophic organisms, such as fungi, that do not require photosynthesis and use nutrients from decaying vegetation. The dominance of fungal species lasted only a few years while the atmosphere cleared and plenty of organic matter to feed on was present. Once the atmosphere cleared, photosynthetic organisms, initially ferns and other ground-level plants, returned.[64] Just two species of fern appear to have dominated the landscape for centuries after the event.[65]
Polyploidy appears to have enhanced the ability of flowering plants to survive the extinction, probably because the additional copies of the genome such plants possessed, allowed them to more readily adapt to the rapidly changing environmental conditions that followed the impact.[66]
AmphibiansEdit
There is limited evidence for extinction of amphibians at the K–Pg boundary. A study of fossil vertebrates across the K–Pg boundary in Montana concluded that no species of amphibian became extinct.[67] Yet there are several species of Maastrichtian amphibian, not included as part of this study, which are unknown from the Paleocene. These include the frog Theatonius lancensis[68] and the albanerpetontid Albanerpeton galaktion;[69] therefore, some amphibians do seem to have become extinct at the boundary. The relatively low levels of extinction seen among amphibians probably reflect the low extinction rates seen in freshwater animals.[70]Non-archosaursEdit

Kronosaurus Hunt, a rendering by Dmitry Bogdanov in 2008. Large marine reptiles, including plesiosaurians such as these, became extinct at the end of the Cretaceous.
ChoristodereEdit
The choristoderes (semi-aquatic archosauromorphs) survived across the K–Pg boundary[27] but would die out in the early Miocene.[71] Studies on Champsosaurus' palatal teeth suggest that there were dietary changes among the various species across the KT event.[72]TurtlesEdit
More than 80% of Cretaceous turtle species passed through the K–Pg boundary. Additionally, all six turtle families in existence at the end of the Cretaceous survived into the Paleogene and are represented by living species.[73]LepidosauriaEdit
The living non-archosaurian reptile taxa, lepidosaurians (snakes, lizards and tuataras), survived across the K–Pg boundary.[27] Living lepidosaurs include the tuataras (the only living rhynchocephalians) and the squamates.The rhynchocephalians were a widespread and relatively successful group of lepidosaurians during the early Mesozoic, but began to decline by the mid-Cretaceous, although they were very successful in the Late Cretaceous of South America.[74] They are represented today by a single genus, located exclusively in New Zealand.[75]
The order Squamata, which is represented today by lizards, snakes and amphisbaenians (worm lizards), radiated into various ecological niches during the Jurassic and was successful throughout the Cretaceous. They survived through the K–Pg boundary and are currently the most successful and diverse group of living reptiles, with more than 6,000 extant species. Many families of terrestrial squamates became extinct at the boundary, such as monstersaurians and polyglyphanodonts, and fossil evidence indicates they suffered very heavy losses in the K–T event, only recovering 10 million years after it.[76] Giant non-archosaurian aquatic reptiles such as mosasaurs and plesiosaurs, which were the top marine predators of their time, became extinct by the end of the Cretaceous.[77][78] The ichthyosaurs had disappeared from fossil records before the mass extinction occurred.
ArchosaursEdit
The archosaur clade includes two surviving groups, crocodilians and birds, along with the various extinct groups of non-avian dinosaurs and pterosaurs.[79]CrocodyliformsEdit
Ten families of crocodilians or their close relatives are represented in the Maastrichtian fossil records, of which five died out prior to the K–Pg boundary.[80] Five families have both Maastrichtian and Paleocene fossil representatives. All of the surviving families of crocodyliforms inhabited freshwater and terrestrial environments—except for the Dyrosauridae, which lived in freshwater and marine locations. Approximately 50% of crocodyliform representatives survived across the K–Pg boundary, the only apparent trend being that no large crocodiles survived.[27] Crocodyliform survivability across the boundary may have resulted from their aquatic niche and ability to burrow, which reduced susceptibility to negative environmental effects at the boundary.[56] Jouve and colleagues suggested in 2008 that juvenile marine crocodyliforms lived in freshwater environments as do modern marine crocodile juveniles, which would have helped them survive where other marine reptiles became extinct; freshwater environments were not so strongly affected by the K–Pg extinction event as marine environments were.[81]PterosaursEdit
One family of pterosaurs, Azhdarchidae, was definitely present in the Maastrichtian, and it likely became extinct at the K–Pg boundary. These large pterosaurs were the last representatives of a declining group that contained ten families during the mid-Cretaceous. Several other pterosaur lineages may have been present during the Maastrichtian, such as the ornithocheirids, pteranodontids, nyctosaurids, as well as, a possible tapejarid, though they are represented by fragmentary remains that are difficult to assign to any given group.[82][83] While this was occurring, modern birds were undergoing diversification; traditionally it was thought that they replaced archaic birds and pterosaur groups, possibly due to direct competition, or they simply filled empty niches,[56][84][85] but there is no correlation between pterosaur and avian diversities that are conclusive to a competition hypothesis,[86] and small pterosaurs were present in the Late Cretaceous.[87] In fact, at least some niches previously held by birds were reclaimed by pterosaurs prior to the K–Pg event.[88]BirdsEdit
Most paleontologists regard birds as the only surviving dinosaurs (see Origin of birds). It is thought that all non-avian theropods became extinct, including then-flourishing groups such as enantiornithines and hesperornithiforms.[89] Several analyses of bird fossils show divergence of species prior to the K–Pg boundary, and that duck, chicken, and ratite bird relatives coexisted with non-avian dinosaurs.[90] Large collections of bird fossils representing a range of different species provides definitive evidence for the persistence of archaic birds to within 300,000 years of the K–Pg boundary. The absence of these birds in the Paleogene is evidence that a mass extinction of archaic birds took place there. A small fraction of the Cretaceous bird species survived the impact, giving rise to today's birds.[15][91] The only bird group known for certain to have survived the K–Pg boundary is the Aves.[15] Avians may have been able to survive the extinction as a result of their abilities to dive, swim, or seek shelter in water and marshlands. Many species of avians can build burrows, or nest in tree holes or termite nests, all of which provided shelter from the environmental effects at the K–Pg boundary. Long-term survival past the boundary was assured as a result of filling ecological niches left empty by extinction of non-avian dinosaurs.[56]Non-avian dinosaursEdit
The growing consensus about the endothermy of dinosaurs (see dinosaur physiology) helps to understand their full extinction in contrast with their close relatives, the crocodilians. Ectothermic ("cold-blooded") crocodiles have very limited needs for food (they can survive several months without eating) while endothermic ("warm-blooded") animals of similar size need much more food to sustain their faster metabolism. Thus, under the circumstances of food chain disruption previously mentioned, non-avian dinosaurs died,[26] while some crocodiles survived. In this context, the survival of other endothermic animals, such as some birds and mammals, could be due, among other reasons, to their smaller needs for food, related to their small size at the extinction epoch.[93]
Whether the extinction occurred gradually or suddenly has been debated, as both views have support from the fossil record. A study of 29 fossil sites in Catalan Pyrenees of Europe in 2010 supports the view that dinosaurs there had great diversity until the asteroid impact, with more than 100 living species.[94] More recent research indicates that this figure is obscured by taphonomical biases, however, and the sparsity of the continental fossil record. The results of this study, which were based on estimated real global biodiversity, showed that between 628 and 1,078 non-avian dinosaur species were alive at the end of the Cretaceous and underwent sudden extinction after the Cretaceous–Paleogene extinction event.[95] Alternatively, interpretation based on the fossil-bearing rocks along the Red Deer River in Alberta, Canada, supports the gradual extinction of non-avian dinosaurs; during the last 10 million years of the Cretaceous layers there, the number of dinosaur species seems to have decreased from about 45 to approximately 12. Other scientists have made the same assessment following their research.[96]
Several researchers support the existence of Paleocene non-avian dinosaurs. Evidence of this existence is based on the discovery of dinosaur remains in the Hell Creek Formation up to 1.3 m (4 ft 3.2 in) above and 40,000 years later than the K–Pg boundary.[97] Pollen samples recovered near a fossilized hadrosaur femur recovered in the Ojo Alamo Sandstone at the San Juan River in Colorado, indicate that the animal lived during the Cenozoic, approximately 64.5 Ma (about 1 million years after the K–Pg extinction event). If their existence past the K–Pg boundary can be confirmed, these hadrosaurids would be considered a dead clade walking.[98] Scientific consensus, however, is that these fossils were eroded from their original locations and then re-buried in much later sediments (also known as reworked fossils).[99]
MammalsEdit
All major Cretaceous mammalian lineages, including monotremes (egg-laying mammals), multituberculates, metatherians, eutherians, dryolestoideans,[100] and gondwanatheres[101] survived the K–Pg extinction event, although they suffered losses. In particular, metatherians largely disappeared from North America, and the Asian deltatheroidans became extinct (aside from the lineage leading to Gurbanodelta).[102] In the Hell Creek beds of North America, at least half of the ten known multituberculate species and all eleven metatherians species, are not found above the boundary.[92] Multituberculates in Europe and North America survived relatively unscathed and quickly bounced back in the Palaeocene, but Asian forms were decimated, never again to represent a significant component on mammalian faunas.[103] A recent study indicates that metatherians suffered the heaviest losses at the K–T event, followed by multituberculates, while eutherians recovered the quickest.[104]Mammalian species began diversifying approximately 30 million years prior to the K–Pg boundary. Diversification of mammals stalled across the boundary.[105] Current research indicates that mammals did not explosively diversify across the K–Pg boundary, despite the environment niches made available by the extinction of dinosaurs.[106] Several mammalian orders have been interpreted as diversifying immediately after the K–Pg boundary, including Chiroptera (bats) and Cetartiodactyla (a diverse group that today includes whales and dolphins and even-toed ungulates),[106] although recent research concludes that only marsupial orders diversified after the K–Pg boundary.[105]
K–Pg boundary mammalian species were generally small, comparable in size to rats; this small size would have helped them find shelter in protected environments. In addition, it is postulated that some early monotremes, marsupials, and placentals were semiaquatic or burrowing, as there are multiple mammalian lineages with such habits today. Any burrowing or semiaquatic mammal would have had additional protection from K–Pg boundary environmental stresses.[56]
EvidenceEdit
North American fossilsEdit
In North American terrestrial sequences, the extinction event is best represented by the marked discrepancy between the rich and relatively abundant late-Maastrichtian palynomorph record and the post-boundary fern spike.[59]At present the most informative sequence of dinosaur-bearing rocks in the world from the K–Pg boundary is found in western North America, particularly the late Maastrichtian-age Hell Creek Formation of Montana. Comparison with the older Judith River Formation (Montana) and Dinosaur Park Formation (Alberta), which both date from approximately 75 Ma, provides information on the changes in dinosaur populations over the last 10 million years of the Cretaceous. However, these fossil beds are geographically limited, covering only part of one continent.[92]
The middle–late Campanian formations show a greater diversity of dinosaurs than any other single group of rocks. The late Maastrichtian rocks contain the largest members of several major clades: Tyrannosaurus, Ankylosaurus, Pachycephalosaurus, Triceratops, and Torosaurus,[107] which suggests food was plentiful immediately prior to the extinction.
In addition to rich dinosaur fossils, there are also plant fossils that illustrate the reduction in plant species across the K–Pg boundary. In the sediments below the K–Pg boundary the dominant plant remains are angiosperm pollen grains, but the boundary layer contains little pollen and is dominated by fern spores.[108] More usual pollen levels gradually resume above the boundary layer. This is reminiscent of areas blighted by modern volcanic eruptions, where the recovery is led by ferns, which are later replaced by larger angiosperm plants.[109]
Marine fossilsEdit
The mass extinction of marine plankton appears to have been abrupt and right at the K–Pg boundary.[110] Ammonite genera became extinct at or near the K–Pg boundary; however, there was a smaller and slower extinction of ammonite genera prior to the boundary that was associated with a late Cretaceous marine regression. The gradual extinction of most inoceramid bivalves began well before the K–Pg boundary, and a small, gradual reduction in ammonite diversity occurred throughout the very late Cretaceous.[111]Further analysis shows that several processes were in progress in the late Cretaceous seas and partially overlapped in time, then ended with the abrupt mass extinction.[111] The diversity of marine life decreased when the climate near the K–Pg boundary increased in temperature. The temperature increased about three to four degrees very rapidly between 65.4 and 65.2 million years ago, which is very near the time of the extinction event. Not only did the climate temperature increase, but the water temperature decreased, causing a drastic decrease in marine diversity.[112]
MegatsunamisEdit
The scientific consensus is that the asteroid impact at the K–Pg boundary left megatsunami deposits and sediments around the area of the Caribbean Sea and Gulf of Mexico, from the colossal waves created by the impact.[113] These deposits have been identified in the La Popa basin in northeastern Mexico,[114] platform carbonates in northeastern Brazil,[115] in Atlantic deep-sea sediments,[116] and in the form of the thickest-known layer of graded sand deposits, around 100 m (330 ft), in the Chicxulub crater itself, directly above the shocked granite ejecta.The megatsunami has been estimated at more than 100 m (330 ft) tall, as the asteroid fell into relatively shallow seas; in deep seas it would have been 4.6 km (2.9 mi) tall.[117]
Fossils in sedimentary rocks deposited during the impactEdit
Recently, fossiliferous sedimentary rocks deposited during the K-T impact have been found in the Gulf of Mexico area, including tsunami wash deposits carrying remains of a mangrove-type life system, evidence that after the impact water sloshed back and forth repeatedly in the Gulf of Mexico, and dead fish left in shallow water but not disturbed by scavengers: "this layer is the K-T boundary". [118][119][120][121][122]DurationEdit
The rapidity of the extinction is a controversial issue, because some theories about the extinction's
causes imply a rapid extinction over a relatively short period (from a
few years to a few thousand years) while others imply longer periods.
The issue is difficult to resolve because of the Signor–Lipps effect; that is, the fossil record is so incomplete that most extinct species probably died out long after the most recent fossil that has been found.[123]
Scientists have also found very few continuous beds of fossil-bearing
rock that cover a time range from several million years before the K–Pg
extinction to a few million years after it.[27]
The sedimentation rate and thickness of K–Pg clay from three sites
suggest rapid extinction, perhaps less than ten thousand years.[124]
At one site in the Denver Basin of Colorado, the 'fern spike' lasted
about one thousand years (no more than 71 thousand years); the earliest
Cenozoic mammals appeared about 185,000 years (no more than 570,000
years) after the K–Pg boundary layer was deposited.[125]
Chicxulub impactEdit
Evidence for impactEdit
In 1980, a team of researchers consisting of Nobel Prize-winning physicist Luis Alvarez, his son, geologist Walter Alvarez, and chemists Frank Asaro and Helen Michel discovered that sedimentary layers found all over the world at the Cretaceous–Paleogene boundary contain a concentration of iridium many times greater than normal (30, 160, and 20 times in three sections originally studied). Iridium is extremely rare in Earth's crust because it is a siderophile element which mostly sank along with iron into Earth's core during planetary differentiation. As iridium remains abundant in most asteroids and comets, the Alvarez team suggested that an asteroid struck the Earth at the time of the K–Pg boundary.[9] There were earlier speculations on the possibility of an impact event, but this was the first hard evidence.[126]
The K–Pg boundary exposure in Trinidad Lake State Park, in the Raton Basin of Colorado, shows an abrupt change from dark- to light-colored rock. White line added to mark the transition.
In a 2013 paper, Paul Renne of the Berkeley Geochronology Center dated the impact at 66.043±0.011 million years ago, based on argon–argon dating. He further posits that the mass extinction occurred within 32,000 years of this date.[3][132]
In 2007, it was proposed that the impactor belonged to the Baptistina family of asteroids.[133] This link has been doubted, though not disproved, in part because of a lack of observations of the asteroid and its family.[134] It was recently discovered that 298 Baptistina does not share the chemical signature of the K–Pg impactor.[135] Further, a 2011 Wide-field Infrared Survey Explorer (WISE) study of reflected light from the asteroids of the family estimated their break-up at 80 Ma, giving them insufficient time to shift orbits and impact Earth by 66 Ma.[136]
Effects of impactEdit
In March 2010, an international panel of 41 scientists reviewed 20 years of scientific literature and endorsed the asteroid hypothesis, specifically the Chicxulub impact, as the cause of the extinction, ruling out other theories such as massive volcanism. They had determined that a 10-to-15-kilometer (6 to 9 mi) asteroid hurtled into Earth at Chicxulub on Mexico's Yucatán Peninsula. The collision would have released the same energy as 100 teratonnes of TNT (420 zettajoules)—more than a billion times the energy of the atomic bombings of Hiroshima and Nagasaki.[12]The Chicxulub impact caused a global catastrophe. Some of the phenomena were brief occurrences immediately following the impact, but there were also long-term geochemical and climatic disruptions that devastated the ecology. Some scientists are, however, of the opinion that the asteroid impact would not have caused a global extinction if it had impacted somewhere else that wasn’t rich in deposits of sulfur, hydrocarbon or organic fossil fuel as present in the Yucatán peninsula. They explain that fossil fuel deposits caused an explosion so great that tons of soot were jetted up into the stratosphere and blotted out the sun for an extended period of time.[137]
The re-entry of ejecta into Earth's atmosphere would include a brief (hours-long) but intense pulse of infrared radiation, cooking exposed organisms.[56] This is debated, however, with opponents arguing that local ferocious fires, probably limited to North America, fall short of global firestorms. This is the "Cretaceous–Palaeogene firestorm debate". A paper in 2013 by a prominent modeler of nuclear winter suggested that, based on the amount of soot in the global debris layer, the entire terrestrial biosphere might have burned, implying a global soot-cloud blocking out the sun and creating an impact winter effect.[138]
Aside from the hypothesized fire and/or impact winter effects, the impact would have created a dust cloud that blocked sunlight for up to a year, inhibiting photosynthesis.[110] The asteroid hit an area of carbonate rock containing a large amount of combustible hydrocarbons and sulphur,[139] much of which was vaporized, thereby injecting sulfuric acid aerosols into the stratosphere, which might have reduced sunlight reaching the Earth's surface by more than 50%, and would have caused acid rain.[110][140] The resulting acidification of the oceans would kill many organisms that grow shells of calcium carbonate. At Brazos section, the sea surface temperature dropped as much as 7 °C (13 °F) for decades after the impact.[141] It would take at least ten years for such aerosols to dissipate, and would account for the extinction of plants and phytoplankton, and subsequently herbivores and their predators. Creatures whose food chains were based on detritus would have a reasonable chance of survival, however.[93][110] Freezing temperatures probably lasted for at least three years.[142]
If widespread fires occurred, they would have increased the CO
2 content of the atmosphere and caused a temporary greenhouse effect once the dust clouds and aerosol settled, and, this would have exterminated the most vulnerable organisms that survived the period immediately after the impact.[143]
Although most paleontologists now agree that an asteroid did hit the Earth at approximately the end of the Cretaceous, there is an ongoing dispute whether the impact was the sole cause of the extinctions.[40][144]

The
river bed at the Moody Creek Mine, 7 Mile Creek / Waimatuku, Dunollie,
New Zealand contains evidence of a devastating event on terrestrial
plant communities at the Cretaceous-Paleogene boundary, confirming the
severity and global nature of the event.[59]
2016 Chicxulub crater drilling projectEdit
In 2016, a scientific drilling project obtained deep rock-core samples from the peak ring around the Chicxulub impact crater. The discoveries confirmed that the rock comprising the peak ring had been shocked by immense pressure and melted in just minutes from its usual state into its present form. Unlike sea-floor deposits, the peak ring was made of granite originating much deeper in the earth, which had been ejected to the surface by the impact. Gypsum is a sulfate-containing rock usually present in the shallow seabed of the region; it had been almost entirely removed, vaporized into the atmosphere. Further, the event was immediately followed by a megatsunami[d] sufficient to lay down the largest known layer of sand separated by grain size directly above the peak ring.These findings strongly support the impact's role in the extinction event. The impactor was large enough to create a 190-kilometer-wide (120 mi) peak ring, to melt, shock, and eject deep granite, to create colossal water movements, and to eject an immense quantity of vaporized rock and sulfates into the atmosphere, where they would have persisted for several years. This worldwide dispersal of dust and sulfates would have affected climate catastrophically, led to large temperature drops, and devastated the food chain.[145][146]
Alternative hypothesesEdit
Although the concurrence of the end-Cretaceous extinctions with the
Chicxulub asteroid impact strongly supports the impact hypothesis, some
scientists continue to support other contributing causes: volcanic
eruptions, climate change, sea level change, and other impact events.
The end-Cretaceous event is the only mass extinction known to be associated with an impact, and other large impacts, such as the Manicouagan Reservoir impact, do not coincide with any noticeable extinction events.[147]
The Deccan Traps could have caused extinction through several mechanisms, including the release of dust and sulfuric aerosols into the air, which might have blocked sunlight and thereby reduced photosynthesis in plants. In addition, Deccan Trap volcanism might have resulted in carbon dioxide emissions that increased the greenhouse effect when the dust and aerosols cleared from the atmosphere.[149][150]
In the years when the Deccan Traps hypothesis was linked to a slower extinction, Luis Alvarez (d. 1988) replied that paleontologists were being misled by sparse data. While his assertion was not initially well-received, later intensive field studies of fossil beds lent weight to his claim. Eventually, most paleontologists began to accept the idea that the mass extinctions at the end of the Cretaceous were largely or at least partly due to a massive Earth impact. Even Walter Alvarez acknowledged that other major changes may have contributed to the extinctions.[151]
Combining these theories, some geophysical models suggest that the impact contributed to the Deccan Traps. These models, combined with high-precision radiometric dating, suggest that the Chicxulub impact could have triggered some of the largest Deccan eruptions, as well as eruptions at active volcanoes anywhere on Earth.[152][153]
A severe regression would have greatly reduced the continental shelf area, the most species-rich part of the sea, and therefore could have been enough to cause a marine mass extinction; however, this change would not have sufficed to cause the extinction of the ammonites. The regression would also have caused climate changes, partly by disrupting winds and ocean currents and partly by reducing the Earth's albedo and increasing global temperatures.[111]
Marine regression also resulted in the loss of epeiric seas, such as the Western Interior Seaway of North America. The loss of these seas greatly altered habitats, removing coastal plains that ten million years before had been host to diverse communities such as are found in rocks of the Dinosaur Park Formation. Another consequence was an expansion of freshwater environments, since continental runoff now had longer distances to travel before reaching oceans. While this change was favorable to freshwater vertebrates, those that prefer marine environments, such as sharks, suffered.[92]
Recent work led by Sierra Peterson at Seymour Island, Antarctica, showed two separate extinction events near the Cretaceous-Paleogene boundary, with one correlating to Deccan Trap volcanism and one correlated with the Chicxulub impact.[159] The team analyzed combined extinction patterns using a new clumped isotope temperature record from a hiatus-free, expanded K–Pg boundary section. They documented a 7.8±3.3 °C warming synchronous with the onset of Deccan Traps volcanism and a second, smaller warming at the time of meteorite impact. They suggest local warming may have been amplified due to simultaneous disappearance of continental or sea ice. Intra-shell variability indicates a possible reduction in seasonality after Deccan eruptions began, continuing through the meteorite event. Species extinction at Seymour Island occurred in two pulses that coincide with the two observed warming events, directly linking the end-Cretaceous extinction at this site to both volcanic and meteorite events via climate change.[159]
Deccan TrapsEdit
Before 2000, arguments that the Deccan Traps flood basalts caused the extinction were usually linked to the view that the extinction was gradual, as the flood basalt events were thought to have started around 68 Mya and lasted more than 2 million years. The most recent evidence shows that the traps erupted over a period of only 800,000 years spanning the K–Pg boundary, and therefore may be responsible for the extinction and the delayed biotic recovery thereafter.[148]The Deccan Traps could have caused extinction through several mechanisms, including the release of dust and sulfuric aerosols into the air, which might have blocked sunlight and thereby reduced photosynthesis in plants. In addition, Deccan Trap volcanism might have resulted in carbon dioxide emissions that increased the greenhouse effect when the dust and aerosols cleared from the atmosphere.[149][150]
In the years when the Deccan Traps hypothesis was linked to a slower extinction, Luis Alvarez (d. 1988) replied that paleontologists were being misled by sparse data. While his assertion was not initially well-received, later intensive field studies of fossil beds lent weight to his claim. Eventually, most paleontologists began to accept the idea that the mass extinctions at the end of the Cretaceous were largely or at least partly due to a massive Earth impact. Even Walter Alvarez acknowledged that other major changes may have contributed to the extinctions.[151]
Combining these theories, some geophysical models suggest that the impact contributed to the Deccan Traps. These models, combined with high-precision radiometric dating, suggest that the Chicxulub impact could have triggered some of the largest Deccan eruptions, as well as eruptions at active volcanoes anywhere on Earth.[152][153]
Multiple impact eventEdit
Other crater-like topographic features have also been proposed as impact craters formed in connection with Cretaceous-Paleogene extinction. This suggests the possibility of near-simultaneous multiple impacts, perhaps from a fragmented asteroidal object similar to the Shoemaker–Levy 9 impact with Jupiter. In addition to the 180 km (110 mi) Chicxulub crater, there is the 24 km (15 mi) Boltysh crater in Ukraine (65.17±0.64 Ma), the 20 km (12 mi) Silverpit crater in the North Sea (59.5±14.5 Ma) possibly formed by bolide impact, and the controversial and much larger 600 km (370 mi) Shiva crater. Any other craters that might have formed in the Tethys Ocean would have been obscured by the northward tectonic drift of Africa and India.[154][155][156][157]Maastrichtian sea-level regressionEdit
There is clear evidence that sea levels fell in the final stage of the Cretaceous by more than at any other time in the Mesozoic era. In some Maastrichtian stage rock layers from various parts of the world, the later layers are terrestrial; earlier layers represent shorelines and the earliest layers represent seabeds. These layers do not show the tilting and distortion associated with mountain building, therefore the likeliest explanation is a regression, a drop in sea level. There is no direct evidence for the cause of the regression, but the currently accepted explanation is that the mid-ocean ridges became less active and sank under their own weight.[27][158]A severe regression would have greatly reduced the continental shelf area, the most species-rich part of the sea, and therefore could have been enough to cause a marine mass extinction; however, this change would not have sufficed to cause the extinction of the ammonites. The regression would also have caused climate changes, partly by disrupting winds and ocean currents and partly by reducing the Earth's albedo and increasing global temperatures.[111]
Marine regression also resulted in the loss of epeiric seas, such as the Western Interior Seaway of North America. The loss of these seas greatly altered habitats, removing coastal plains that ten million years before had been host to diverse communities such as are found in rocks of the Dinosaur Park Formation. Another consequence was an expansion of freshwater environments, since continental runoff now had longer distances to travel before reaching oceans. While this change was favorable to freshwater vertebrates, those that prefer marine environments, such as sharks, suffered.[92]
Multiple causesEdit
Proponents of multiple causation view the suggested single causes as either too small to produce the vast scale of the extinction, or not likely to produce its observed taxonomic pattern.[92] In a review article, J. David Archibald and David E. Fastovsky discussed a scenario combining three major postulated causes: volcanism, marine regression, and extraterrestrial impact. In this scenario, terrestrial and marine communities were stressed by the changes in, and loss of, habitats. Dinosaurs, as the largest vertebrates, were the first affected by environmental changes, and their diversity declined. At the same time, particulate materials from volcanism cooled and dried areas of the globe. Then an impact event occurred, causing collapses in photosynthesis-based food chains, both in the already-stressed terrestrial food chains and in the marine food chains.Recent work led by Sierra Peterson at Seymour Island, Antarctica, showed two separate extinction events near the Cretaceous-Paleogene boundary, with one correlating to Deccan Trap volcanism and one correlated with the Chicxulub impact.[159] The team analyzed combined extinction patterns using a new clumped isotope temperature record from a hiatus-free, expanded K–Pg boundary section. They documented a 7.8±3.3 °C warming synchronous with the onset of Deccan Traps volcanism and a second, smaller warming at the time of meteorite impact. They suggest local warming may have been amplified due to simultaneous disappearance of continental or sea ice. Intra-shell variability indicates a possible reduction in seasonality after Deccan eruptions began, continuing through the meteorite event. Species extinction at Seymour Island occurred in two pulses that coincide with the two observed warming events, directly linking the end-Cretaceous extinction at this site to both volcanic and meteorite events via climate change.[159]
Recovery and radiationEdit

An artist's rendering of Thescelosaurus shortly after the K-Pg mass extinction. It survived by burrowing, but would soon die of starvation.
Other groups also underwent major radiations. Based on molecular sequencing and fossil dating, Neoaves appeared to radiate after the K–Pg boundary.[23][161] They even produced giant, flightless forms, such as the herbivorous Gastornis and Dromornithidae, and the predatory Phorusrhacidae. The extinction of Cretaceous lizards and snakes may have led to the radiation of modern groups such as iguanas, monitor lizards, and boas.[16] On land, giant boid and enormous madtsoiid snakes appeared, and in the seas, giant sea snakes radiated. Teleost fish diversified explosively,[24] filling the niches left vacant by the extinction. Groups appearing in the Paleocene and Eocene include billfish, tunas, eels, and flatfish. Major changes are also seen in Paleogene insect communities. Many groups of ants were present in the Cretaceous, but in the Eocene ants became dominant and diverse, with larger colonies. Butterflies diversified as well, perhaps to take the place of leaf-eating insects wiped out by the extinction. The advanced mound-building termites, Termitidae, also appear to have risen in importance.[162]
See alsoEdit
- Climate across Cretaceous–Paleogene boundary
- Late Devonian extinction
- Life timeline
- List of possible impact structures on Earth
- Nature timeline
- Ordovician–Silurian extinction events
- Permian–Triassic extinction event
- Timeline of Cretaceous-Paleogene extinction event research
- Triassic–Jurassic extinction event
NotesEdit
- A megatsunami is a massive movement of sea waters, which can reach inland tens or hundreds of kilometers.
ReferencesEdit
- Grimaldi, David A. (2007). Evolution of the Insects. Cambridge Univ Pr (E). ISBN 978-0-511-12388-7.
Further readingEdit
- Fortey, Richard (2005). Earth: An Intimate History. New York: Vintage Books. ISBN 978-0-375-70620-2. OCLC 54537112.
- Douglas Preston, "The Day the Earth Died: A young paleontologist makes the find of his life", The New Yorker, 8 April 2019, pp. 52–65. Robert A. De Palma has found strong evidence that the dinosaurs—and nearly all other life on Earth—were indeed wiped out 66 million years ago by the Chicxulub asteroid. [1]
External linksEdit
| Wikimedia Commons has media related to K/T Event. |
- "The Great Chicxulub Debate 2004". Geological Society of London. 2004. Retrieved 2007-08-02.
- Kring DA (2005). "Chicxulub Impact Event: Understanding the K–T Boundary". NASA Space Imagery Center. Archived from the original on June 29, 2007. Retrieved 2007-08-02.
- Cowen R (2000). "The K–T extinction". University of California Museum of Paleontology. Retrieved 2007-08-02.
- "What killed the dinosaurs?". University of California Museum of Paleontology. 1995. Retrieved 2007-08-02.
- Papers and presentations resulting from the 2016 Chicxulub drilling project
- De Palma, Robert A.; et al. (1 April 2019). "A seismically induced onshore surge deposit at the KPg boundary, North Dakota". PNAS. doi:10.1073/pnas.1817407116.</ref>
Orion correlation theory
Representation
of the central tenet of the Orion Correlation Theory: the outline of
the Giza pyramids superimposed over a photograph of the stars in Orion's
Belt. The validity of this match has been called into question by
Hancock's critics.
It posits that there is a correlation between the location of the three largest pyramids of the Giza pyramid complex and Orion's Belt of the constellation Orion, and that this correlation was intended as such by the original builders of the Giza pyramid complex. The stars of Orion were associated with Osiris, the god of rebirth and afterlife by the ancient Egyptians.[2][3][4] Depending on the version of the theory, additional pyramids can be included to complete the picture of the Orion constellation, and the Nile river can be included to match with the Milky Way galaxy. The theory was first published in 1989 in Discussions in Egyptology, volume 13. It was the subject of a bestseller, The Orion Mystery, in 1994,[5] as well as a BBC documentary, The Great Pyramid: Gateway to the Stars (February 1994), and appears in some new-age books.[6][7]
History
The Orion correlation theory was put forward by Robert Bauval, and mentioned that Mintaka, the dimmest and most westerly of the stars making up Orion's belt, was offset slightly from the others. Bauval then made a connection between the layout of the three main stars in Orion's belt and the layout of the three main pyramids in the Giza pyramid complex. He published this idea in 1977 in the journal Discussions in Egyptology, volume 13. The idea has been further expounded by Bauval in collaboration with Adrian Gilbert (The Orion Mystery, 1994) and Graham Hancock (Keeper of Genesis, 1996), as well as in their separate publications. The basis of this theory concerns the proposition that the relative positions of three main Ancient Egyptian pyramids on the Giza plateau was by design correlated with the relative positions of the three stars in the constellation of Orion which make up Orion's Belt— as these stars appeared in 10,000 BC.Their initial ideas regarding the alignment of the Giza pyramids with Orion ("…the three pyramids were a terrestrial map of the three stars of Orion's belt"— Hancock's Fingerprints of the Gods, 1995, p. 375) are later joined with speculation about the age of the Great Sphinx (Hancock and Bauval, Keeper of Genesis, published 1996, and in 1997 in the U.S. as The Message of the Sphinx). According to these works, the Great Sphinx was constructed c. 10,500 BC (Upper Paleolithic), and its lion-shape is maintained to be a definitive reference to the constellation of Leo. Furthermore, the orientation and dispositions of the Sphinx, the Giza pyramids and the Nile River relative to one another on the ground is put forward as an accurate reflection or "map" of the constellations of Leo, Orion (specifically, Orion's Belt) and the Milky Way respectively. As Hancock puts it in 1998's The Mars Mystery[8] (co-authored with Bauval):
...we have demonstrated with a substantial body of evidence that the pattern of stars that is "frozen" on the ground at Giza in the form of the three pyramids and the Sphinx represents the disposition of the constellations of Orion and Leo as they looked at the moment of sunrise on the spring equinox during the astronomical "Age of Leo" (i.e., the epoch in which the Sun was "housed" by Leo on the spring equinox.) Like all precessional ages this was a 2,160-year period. It is generally calculated to have fallen between the Gregorian calendar dates of 10,970 and 8810 BC.[8]The allusions to dates circa 12,500 years ago are significant to Hancock since this is the era he seeks to assign to the advanced progenitor civilization, now vanished, but which he contends through most of his works had existed and whose advanced technology influenced and shaped the development of the world's known civilizations of antiquity. Egyptology and archaeological science maintain that available evidence indicates that the Giza pyramids were constructed during the Fourth dynasty period (3rd millennium BC[9]), while the exact date of the Great Sphinx is still unclear. Hancock does not dispute the dating evidence for the currently existing pyramids, but instead argues that they may have been an architectural evolution of sites whose origin and cultural significance dated back some eight thousand years before the current monuments were built —since the Orion correlation theory argues they are oriented that way— which, it is implied, provides further evidence for the influence of astronomical, mathematical, and historical knowledge that might not have been passed down to the pyramids’ builders.
Critique
Arguments made by Hancock, Bauval, Anthony West and others concerning the significance of the proposed correlations have been critiqued.Among these are critiques from two astronomers, Ed Krupp of Griffith Observatory in Los Angeles and Tony Fairall of the University of Cape Town, South Africa. Using planetarium equipment, Krupp and Fairall independently investigated the angle between the alignment of Orion's Belt and north during the era cited by Hancock, Bauval, et al. (which differs from the angle seen today or in the third millennium BC, because of the precession of the equinoxes). They found that the angle was somewhat different from the "perfect match" thought to exist by Bauval and Hancock in the Orion Constellation Theory. They estimate 47–50 degrees per the planetarium measurements, compared to the 38-degree angle formed by the pyramids.[10]
Krupp pointed out that the slightly bent line formed by the three pyramids was deviated towards the north, whereas the slight "kink" in the line of Orion's Belt was deformed to the south, and to match them up one or the other of them had to be turned upside-down.[11] Indeed, this is what was done in the original book by Bauval and Gilbert (The Orion Mystery),[12] which compares images of the pyramids and Orion without revealing that the pyramids’ map had been inverted.[13] Krupp and Fairall find other problems with their arguments, including noting that if the Sphinx is meant to represent the constellation of Leo, then it should be on the opposite side of the Nile (the "Milky Way") from the pyramids ("Orion"),[10][11] that the vernal equinox c. 10,500 BC was in Virgo and not Leo,[10] and that in any case the constellations of the Zodiac originate from Mesopotamia and were completely unknown in Egypt until the much later Graeco-Roman era.[13] Ed Krupp repeated this "upside down" statement in the BBC documentary Atlantis Reborn (1999).
Bauval stated that some astronomers including Archie Roy of the University of Glasgow have rejected Krupp's argument. Krupp presented a counterpoint to the objections, that Bauval stated had been made by the late Dr. Roy, who was a Professor Emeritus of Astronomy at Glasgow University (including the accusation that Bauval and Gilbert deliberately inverted the pyramid map).[clarification needed][13][not in citation given].[14]
In a ruling by the Broadcasting Standards Commission (UK), the committee ruled in favour of Robert Bauval that Krupp's statement that maps were placed upside down was "unfairly" presented in the BBC documentary Atlantis Reborn, without including Bauval's filmed response.[1] Bauval and Hancock's filmed responses to Krupp's statements were included in the modified version of the documentary Atlantis Reborn Again shown on 14 December 2000.
Andrew Collins and Rodney Hale have proposed an 'alternative correlation theory' using three bright stars in the Cygnus constellation.[15] They argued that not only did the groundplan fit the sky but also the stars can be observed setting down on the corresponding pyramids. Their proposal has also been contested.[16]
Leo and the Sphinx
The Great Sphinx is a colossal statue with the face of a man and the body of a lion. Carved out of the surrounding limestone bedrock, it is 57 metres (187 ft) long, 6 metres (20 ft) wide, and has a height of 20 metres (66 ft), making it the largest single-stone statue in the world. The Great Sphinx is one of the world's largest and oldest statues, yet basic facts about it such as the real-life model for the face, when and why it was built, and by whom, are debated. These questions have collectively earned the title "Riddle of the Sphinx", a nod to its Greek namesake.The Great Sphinx is commonly accepted by Egyptologists to represent the likeness of King Khafra (also known by the Hellenised version of his name, Chephren)[17] who is often credited as the builder as well. This would place the time of construction somewhere between 2520 BC and 2494 BC. Because the limited evidence giving provenance to Khafra is ambiguous, the idea of who built the Sphinx, and when, continues to be the subject of debate. An argument put forward by Bauval and Hancock to support the Orion Correlation Theory is that the construction of the Great Sphinx was begun in 10,500 BC; that the Sphinx's lion-shape is a definitive reference to the constellation of Leo; and that the layout and orientation of the Sphinx, the Giza pyramid complex and the Nile River are an accurate reflection or "map" of the constellations of Leo, Orion (specifically, Orion's Belt) and the Milky Way, respectively.[18]
A date of 10,500 BC is chosen because they maintain this is the only time in the precession of the equinoxes when the astrological age was Leo and when that constellation rose directly east of the Sphinx at the vernal equinox. They also suggest that in this epoch the angles between the three stars of Orion's Belt and the horizon were an "exact match" to the angles between the three main Giza pyramids. These propositions and other theories are used to support the overall belief in an advanced and ancient, but now vanished, global progenitor civilization.
The theory that the Sphinx is actually far older has received some support from geologists. Robert M. Schoch has argued that the effects of water erosion on the Sphinx and its surrounding enclosure mean that parts of the monument must originally have been carved at the latest between 7000–5000 BC.[19] Colin Reader has suggested a date only several hundred years prior to the commonly accepted date for construction. These views have been almost universally rejected by mainstream Egyptologists who, together with a number of geologists including James Harrell, Lal Gauri, John J. Sinai, and Jayanta K. Bandyopadhyay,[20][21] stand by the conventional dating for the monument. Their analyses attribute the apparently accelerated wear on the Sphinx variously to modern industrial pollution, qualitative differences between the layers of limestone in the monument itself, scouring by wind-borne sand, or temperature changes causing the stone to crack.
References
- James A. Harrell, "The Sphinx Controversy: Another Look at the Geological Evidence," KMT: A Modern Journal of Ancient Egypt, Vol. 5, No. 2 (Summer 1994), pp. 70–74.
External links
| Wikimedia Commons has media related to Orion Correlation Theory. |



















No comments:
Post a Comment